Hearts, Artificial. The Novacor fully implantable ventricular assist device
Heart disease is a prominent cause of death in affluent nations. In the U.S., it is the number one killer and accounts for more than 39 percent of all deaths. Treatment for heart failure includes medical therapy, surgical intervention, cardiac transplantation, and the use of cardiac devices. Cardiac devices such as heart valves, pacemakers, defibrillators, and intra-aortic balloon pumps can assist the heart, and an artificial heart can replace it.
There are two groups of artificial hearts: total artificial hearts and partial artificial hearts. The total artificial heart (TAH) replaces the failing ventricles (pumping chambers) of a damaged human heart, which is excised or removed from the body. A partial artificial heart or ventricular assist device (VAD) attaches to a failing heart (which remains in the body) and serves to assist in the pumping function of the heart.
The main components of both TAHs and VADs are the energy source, drive-control unit, actuator, and pump. Historically, the actuation of artificial hearts has included pneumatic, hydraulic, electrical (electrohydraulic, electromechanical, electromagnet) and nuclear (thermoenergy) power. Nuclear artificial heart development was halted due to individual and societal concerns relating to the plutonium radioisotope power source.
The first artificial hearts were constructed out of polyvinylchloride, which tested well for endurance, and later silastic and polyurethane materials. Improved biomaterials, particularly compatible blood surface materials, continue to be developed. Over time, both total and partial artificial hearts have changed from large devices situated outside the body (paracorporeal and extracorporeal) to smaller devices intended to be placed inside the body (intracorporeal and fully implantable).
Willem Kolff and Michael DeBakey are among the pioneers in the field of artificial heart development. Kolff invented the artificial kidney machine in Nazi-occupied Holland in 1943. After the war, Kolff led an artificial organ research program at the Cleveland Clinic in Cleveland, Ohio that encouraged research into artificial kidneys, eyes, ears, arms, lungs, and hearts.
In 1957, Kolff and Dr. Tetsuzo Akutsu successfully implanted a crude air-driven artificial heart, composed of two polyvinylchloride pumps, in a dog and maintained circulation for ninety minutes. The Cleveland Clinic group experimented with hydraulically- activated, electromechanical and pneumatic-driven TAHs, and later various types of VADs.
At the Baylor College of Medicine in Houston, Texas, DeBakey and his research team developed a different type of TAH. Their device consisted of two pneumatically-driven silastic sac-type pumps implanted for biventricular bypass. The Baylor College of Medicine group also expanded their research into VADs, heart valves and vascular prostheses.
DeBakey was instrumental in lobbying for the formation of the U.S. Artificial Heart program, established in 1964, at the National Heart Institute of the National Institutes of Health (NIH) in Bethesda, Maryland. The NIH became an important funding source and catalyst for artificial heart research. Under the initial direction of Frank Hastings, the NIH artificial heart program supported investigator research grants and industry contracts for the development of assist and replacement devices totaling more than $400 million. Outside of the U.S., artificial heart research programs were also established in Germany, the former Soviet Union, Japan, and other countries whose research teams experimented with various TAH and VAD prototypes.
By the late 1960s, there were four institutions in the U.S. working on developing TAHs for clinical application: Willem Kolff’s team, now at the University of Utah in Salt Lake City, Utah; the Baylor College of Medicine group headed by Michael DeBakey; William Pierce and his research team at Pennsylvania State University; and the Cleveland Clinic under the direction of Yukihiko Nose;. These researchers contributed incremental improvements on device designs, materials, performance, durability and outcome measures through extensive animal experiments.
The device used in the first clinical case was the pneumatic Liotta TAH, developed by Domingo Liotta and tested in the Baylor College surgical laboratory on calves. At the Texas Heart Institute, Denton Cooley implanted the device as a bridge- to-transplantation in 47-year-old Haskell Karp who could not be weaned from cardiopulmonary bypass after heart surgery. The Liotta TAH sustained Karp for 64 hours, at which time the patient underwent cardiac transplantation. In 1981, Cooley implanted the pneumatic Akutsu III TAH, developed by Tetsuzo Akutsu, which provided a patient with 39 hours of support as a bridge-to-transplantation. Both device implant cases were technical successes, but both patients died shortly after the transplant surgery.
In 1982, William DeVries implanted the pneumatic Jarvik-7 TAH, developed by Robert Jarvik and fellow researchers at the University of Utah, in Barney Clark. Ineligible for cardiac transplantation, Clark consented to the TAH as a permanent implant.
During his 112 days with the TAH, Clark experienced numerous complications including device problems, nasal bleeding and neurological incidents. In 1984 and 1985, three more patients in the U.S. and one in Sweden received permanent Jarvik-7 TAH implants, surviving from 10 to 620 days. In response to severe patient complications, the U.S. Food and Drug Administration suspended Jarvik-7 TAH production and its use as a permanent device in 1991. The Jarvik-7 TAH (renamed Symbion, later CardioWest) continued however to be implanted in patients as bridge-to- transplantation with moderate success rates.
Knowledge gained in TAH research contributed to VAD advancements. VADs experienced success as short-term mechanical support systems, bridge- to-transplantation, and possibly permanent cardiac devices. DeBakey’s research group developed a pneumatic paracorporeal (outside the body) left ventricular assist device (LVAD) for short-term ventricular assistance. In 1967, the first clinical case was reported in which a patient, unable to be weaned from cardiopulmonary bypass, temporarily utilized the LVAD and then had it removed upon cardiac recovery.
By the mid-1970s, clinical trials were underway at the Texas Heart Institute and Boston Children’s Hospital to evaluate the intracorporeal pneumatic VAD—the TECO Model VII—as both a temporary recovery measure and bridge-to-transplantation. In 1984, the electrically powered left ventricle assist system—the Novacor LVAS—emerged as both the first implantable system and successful bridge-to-transplant clinical case (Figure 1).
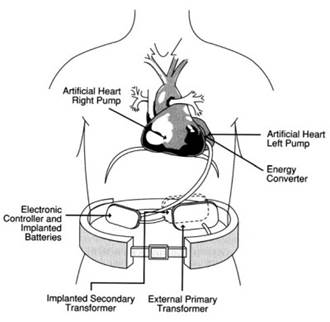
Figure 1. The Novacor fully implantable ventricular assist device. [Used with permission from The Artificial Heart: Prototypes, Policies, and Patients, by the National Academy of Sciences, courtesy of the National Academy Press, Washington D.C., 1991.]
In that same year, the paracorporeal, pneumatic Thoratec VAD also reported a successful bridge-to-transplantation case. Further refinements in the 1990s brought forth wearable ventricular assist systems, which allowed patients to resume basic activities. Clinical trials also began to evaluate VADs for permanent use in patients ineligible for cardiac transplantation.
In 2001, clinical trials with the electromechanical AbioCor TAH as a permanent device began in the U.S. The AbioCor TAH is the first fully implantable device; its components of the replacement heart (hydraulically-driven pump), controller and rechargeable internal battery are implanted in the patient’s chest and abdomen. The AbioCor TAH utilizes a transcutaneous energy transmission (TET) device as a wireless energy transfer system without need for percutaneous lines risking infection.
Artificial heart research encompasses the development of total artificial hearts (TAHs) (see Figure 2) and partial artificial hearts (VADs). In both cases, these devices became more sophisticated, smaller in size, and fully implantable. Both devices were explored as temporary and permanent therapies. Whereas the TAH remains an experimental device, several ventricular assist systems are now FDA-approved for clinical use.
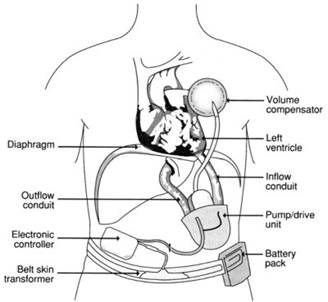
Figure 2. A fully implantable total artificial heart. [Used with permission from The Artificial Heart: Prototypes, Policies, and Patients, by the National Academy of Sciences, courtesy of the National Academy Press, Washington D.C., 1991.]
Not without debate, artificial heart technologies have raised contentious issues of access, quality of life, cost-benefit and reimbursement. The broader context is the role that artificial heart technologies may play towards continuing or challenging the technological imperative in medicine.
Date added: 2023-10-26; views: 684;
