The Hydrologic Cycle. A Schematic Illustration
Water covers two-thirds of the Earth’s surface and, as described above, it is essential for life on Earth. The hydrologic cycle is the complex system whereby water circulates at and near the Earth’s surface, along flow paths, which connect reservoirs. Solar radiation, which is the greatest source of energy on Earth, works with the Earth’s internal energy and gravity to drive the circulation of water through the hydrologic cycle.
The hydrologic cycle is thus driven by and closely coupled to the Earth’s energy cycle. As water circulates through the hydrologic cycle, latent heat exchange plays an important role in the Earth’s surface heat balance.
Most of the Earth’s water - 97% - is in the oceans. The two other major reservoirs of the hydrologic cycle are water associated with land (3%) and the atmosphere (0.001%) (Figure 1). Most of the water associated with land is ice and snow, and most of that is in the polar icecaps. Additional reservoirs of water associated with land include underground water (groundwater, soil pore water, and capillary fringe water above the water table) and surface water (rivers, lakes, and wetlands). A small percentage of water is also present in biota.
Over the Earth as a whole, average annual precipitation and evaporation are each -1.0myr-1 (Figure 1). However, there are important differences in precipitation (P) and evaporation/evapotranspiration (E) over land surfaces versus the oceans. Over the land surfaces, average P is ~0.80 myr-1, but average P over the oceans is somewhat higher, ~ 1.1 myr-1.
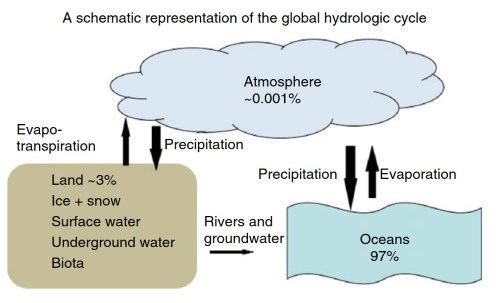
Figure 1. A schematic illustration showing the three main reservoirs of the hydrologic cycle (water in each as %). Major flow paths appear as arrows
Evaporation and evapotranspiration over the land surfaces, E~0.5myr-1, whereas ocean evaporation E~ 1.2myr-1. P and E are approximately balanced over the oceans but P-E over land surfaces equals about 0.3 myr-1. The excess in P versus E over land surfaces leads to a net transfer of water from the oceans to the continents. However, water is returned to the oceans via rivers, along with a small amount of direct discharge of groundwater to oceans in some coastal regions.
The mean residence time (MRT), which is the amount of time that the average drop of water spends between entering and leaving a given reservoir, varies for different components of the hydrologic cycle. Water in the atmosphere has MRT of only 2-3 days, shallow groundwater from days to years, deep groundwater as much as thousands or even millions of years. Water in the shallow oceans has MRT of weeks to months whereas deeper oceans have MRTs of thousands of years.
The average chemical characteristics of water in the various reservoirs of the hydrologic cycle vary considerably. Ocean water has a mean salinity of ~35 ppt (parts per thousand) and major dissolved constituents include Na and Cl along with Mg, SO4, Ca, K, and HCO3 (bicarbonate ion). The average pH of ocean surface water is around 8. In contrast, water in the atmosphere is orders of magnitude more dilute. Atmospheric water is derived primarily from evaporation and transpiration, and when water evaporates, it leaves its dissolved constituents behind.
The major sources of chemical components in the atmosphere include natural aerosols in the form of sea salts, terrestrially derived particles, components released by forest and grassland burning, exhaust from factories and vehicles, gases and particles released by volcanic activity, along with a variety of other components. Absolutely pure water in equilibrium with the atmosphere would have a somewhat acidic pH because reaction with atmospheric CO2 forms carbonic acid.
In many parts of the world, sulfur dioxide (SO2) and nitrogen oxides (NOx) released by the burning of fossil fuels result in sulfuric acid and nitric acid in rainfall, leading to acid rain. However, in some parts of the world, alkaline particles in the atmosphere can drive the pH of rain higher.
When water passes into the underground environment to become soil water and/or ultimately groundwater, the concentrations of chemical constituents tend to be concentrated through evaporation and evapotranspiration. Biological activity within the soil zone can contribute CO2 and natural organic acids, which make the water more acidic and promote mineral dissolution. Other processes such as ion exchange and microbially mediated redox reactions also can impact the water composition.
In groundwaters, Ca, Mg, Na, K, SO4, Cl, and HCO3 tend to make up nearly 95% of the dissolved ions [4]. The chemical composition of groundwater can vary dramatically from one region to another, particularly depending upon whether the rock types consist of more reactive minerals such as carbonates or less reactive minerals such as feldspars and quartz. The chemical composition of groundwater can also vary depending upon the flow rate of water in the particular subsurface environment along with a host of other factors.
Surface waters include rivers, wetlands, and lakes. Rivers transport solutes and sediments, ultimately from the land surfaces to the oceans. The concentrations of dissolved constituents in rivers can vary by over three orders of magnitude in terms of both concentrations and yields (discharge weighted). The average river water concentration of Cl is about 3 orders of magnitude less than that of average ocean water, and one to several orders of magnitude greater than that of precipitation.
Rivers and streams can also carry particles, either lighter materials in suspension or heavier grains along the riverbed. The suspended loads of rivers are the dominant means of carrying chemical constituents on a worldwide basis, and the total suspended load delivered by rivers to the oceans has been estimated at 13.5 X 1091 yr-1.
Date added: 2023-10-27; views: 617;
