The Move to Larger Proteins
As important as these studies were, it was clear that insulin was a relatively small protein (in some estimations it was on the borderline between a peptide and protein) and the elucidation of its sequence had been greatly facilitated by the fact that it was isolated in two smaller pieces (after the disulfides were cleaved by performic acid oxidation) and that it lacked methionine and tryptophan, two amino acids that can present problems during analytical manipulations.
Fortunately there were two additional technological advances 'in the wings' that proved to be essential in allowing the application of Sanger's methods and strategy to larger and more complicated proteins. The first of these was a highly reproducible analytical method for doing amino acid analyses (Moore and Stein, 1993) and the second was a different method for tagging and determining the N-terminal residue of a peptide that could be applied sequentially (Edman, 1950).
Both of these key contributions were in fact already being developed during the time Sanger was sequencing insulin but neither were fully adopted until after he had completed that task.
The development of the automated amino acid analyzer was the work of Stein and Moore and their colleagues (Moore and Stein, 1993) and utilized ion-exchange chromatography for separation and the ninhydrin reagent for detection and quantification. The resin that was finally adopted for this purpose was a sulfonated cross-linked polystyrene known as Dowex 50.
In addition to its ion-exchange properties, it had considerable hydrophobic character that allowed the separation of the nonpolar amino acids as well as the ones bearing polar side chains and provided quantitative identification on appropriately calibrated instruments.
In the early stages of development, a single analysis required about 24 h but constant improvements eventually shortened the time to less than an hour. Coincidently, as a part of this work, they also developed the fraction collector (Stein and Moore, 1948), which is still, in its various manifestations, a staple of any biochemistry laboratory. Both the analyzer and the fraction collector were subsequently developed commercially.
The new N-terminal method, first developed for this purpose by Edman (1950), was based on the reaction of the α-amino group of a peptide with phenyl isothiocyanate in dilute alkali, which produced a phenylthiocarbamyl derivative. The modified peptide would, on exposure to acid, cyclize to a substituted anilinothiazolinone that removed the first residue from the remainder of the peptide. Subsequent conversion of the unstable thiazolinone to the corresponding phenyl thio-hydantoin (commonly referred to as a PTH-amino acid) allowed for its identification by a variety of analytical techniques.
Under conditions that limited the amount of acid cleavage of other peptide bonds in the remainder of the peptide during cyclization, the reaction could be repeated to identify the second amino acid (now the new N-terminus) and so on (Figure 1). When originally applied to isolated peptides derived from enzymatic digests (that were generally in the range of 2-20 amino acid residues), it could also be carried out in a subtractive mode (Hirs et al., 1960) whereby the composition of the peptide was determined before and after each cycle of the Edman degradation to determine what amino acid had been removed (by difference).
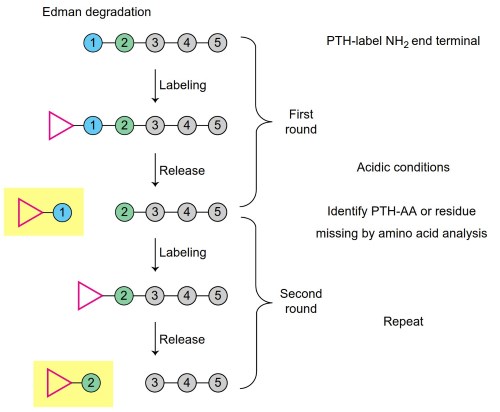
Figure 1. Schematic diagram of sequential cycles of Edman degradation
The availability of high-speed, quantitative amino acid analyses made this a particularly popular approach in the 1960s and early 1970s. The usefulness of the Edman degradation to sequencing studies was greatly enhanced by the adoption of (nearly) anhydrous trifluoroacetic acid (or similar compounds) that allowed cyclization to occur with only trace amounts of background hydrolysis (and minimal degradation of some amino acids) (Konigsberg and Hill, 1962). This was particularly true when this invaluable method was subsequently also automated (Begg and Edman, 1967).
Date added: 2024-06-13; views: 524;
