Intracellular Signaling Lipids Interact with Proteins
Second messenger lipids are produced as a direct or indirect consequence of the stimulation of a vast array of extracellular receptors. The archetypal example of lipid signaling is the PI-PLC-mediated cleavage of PI(4,5)P2 to produce IP3 (which increases intracellular calcium) and the bioactive lipid DAG - a strong activator of PKC (and other) enzymes.
The most common mechanism of lipid-induced protein activation is through interaction of the lipid headgroup with a structural motif on the effector protein often referred to as the lipidbinding domain (LBD). The lipid-binding protein is also referred to as an effector protein because it propagates the signal initiated at the membrane receptor.
LBD - Lipid interactions typically involve the lipid head- group, backbone, and one or more of the fatty acid moieties, but can also include some of the fatty acid chain itself. Fatty acid binding may indeed be a driving factor in ceramide and C1P associations with their protein partners.
However, it is more common that the fatty acid physiochemistry facilitates binding indirectly by altering the lipid headgroup positioning within the membrane, or by promoting the formation of lipid domains that regionally concentrate protein partners and/or alter membrane properties (Lipowsky, 2002; Bigay and Antonny, 2012).
Proteomics studies have identified thousands of LBD- containing proteins in organisms from bacteria through primates. By rough calculation, approximately 6-8% (Based on the assumption that there are 20 000 protein-coding genes in the human genome, and using reported numbers of lipidbinding proteins published by the Lipid MAPs Consortium or by manual summation of LBDs extracted from the EBI InterPro database.) of the protein-coding genes in the human genome contain one or more LBDs, and the majority of these have reported signaling functions (Hunter et al., 2012).
This is likely to be an underestimate based on the observation that some LBDs, such as those that bind PtdOH, ceramide, and C1P, do not contain a readily identifiable lipid-binding motif, but may rely on more general biophysical characteristic(s) of the folded protein for lipid recognition. LBDs identified in proteins using InterPro are shown in Table 2, along with the number of human proteins predicted to contain each one.
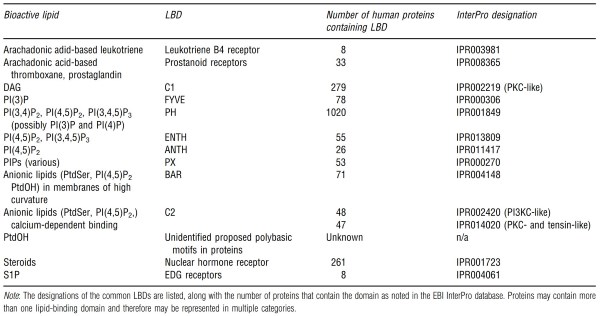
Table 2. Lipid-binding domains (LBDs) in the human genome
Note that some proteins contain more than one type of LBD and may be represented multiple times in this table. The abundance of phosphatidylinositol binding proteins is conspicuous, underscoring the dominance of the phosphatidylinositols as second messenger lipid signals. Indeed, the PH domain category can be subdivided based on empirically determined specificities for various phosphoinositide headgroups. For example, the PH domain in Akt binds PI(3,4)P2 and PI(3,4,5)P3 (see Rong et al., 2001), while the PH domain of phospholipase GS binds PI(4,5)P2 (Garcia etal., 1995).
However, this specificity is not always evident in the primary sequence of a protein, which limits in silico detection of lipid-binding domains. This has resulted in a stumbling block in the characterization of lipid-signaling networks since empirical methods for detecting lipid-binding domains are often laborious and expensive.
In fact, several lipids appear to interact with proteins primarily through nonspecific electrostatic effects. This is the suggested mechanism for signaling through PtdOH and G1P, which are presumed to bind to proteins via a positively charged patch on the protein's threedimensional structure.
General Traits of Biological Membranes
In currently living organisms, cellular membranes are extremely complex in composition, using a repertoire of hundreds if not thousands of lipid species (Figure 1). One of the most detailed pictures of a real biological membrane comes from studies of synaptic vesicles, which encapsulate neurotransmitters and are at the basis of communication between neurons (Takamori et al., 2006).
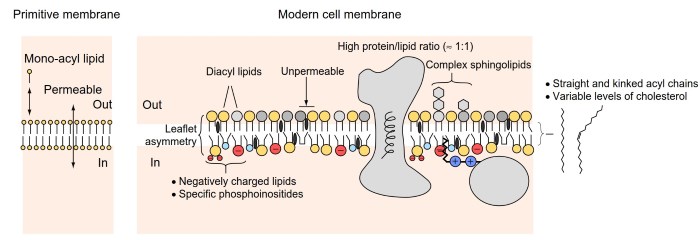
Figure 1. From primitive to modern cell membranes. Primitive cellular membranes might have formed from the self-assembly of simple molecules containing one hydrocarbon acyl chain. These membranes are highly permeable and their stability requires continuous exchange with the environment. The ability to synthesize diacyl lipid was probably a turning point to make membranes more stable and therefore less dependent on the environment.
In turn, this transition would have required the emergence of intracellular mechanisms to control exchanges across the bilayer and to promote membrane division. Modern cell membranes are extremely complex.
They are very rich in transmembrane and peripheral proteins, they contain hundreds of different lipid species, and their two leaflets can be very different in composition. The composition of both bulk and rare lipids is also different between cellular organelles
Comprehensive lipid and protein analysis (i.e., proteomic and lipidomic studies) reveal a very crowded membrane with numerous proteins of different structure and function and about 50 different lipid species. This complexity is intimidating and raises a central question: Do all details matter or can we extract some general traits that distinguish or unify membrane-bound organelles?
The abundance of transmembrane proteins in the synaptic vesicle is not unique. Although the bilayer structure is governed by the amphiphilic properties of lipids, biological membranes generally contain high amounts of proteins (Engelman, 2005); some of them cross the bilayers through transmembrane helical segments; others are peripherally associated through various means.
This crowding probably influences the ability of proteins to move laterally (diffusion), modifies the mechanical properties of membranes, and can lead to membrane heterogeneities.
The building blocks of cellular membranes are glycer- ophospholipids, sphingolipids, and sterols (Holthuis and Levine, 2005). Glycerophospholipids encompass both bulk lipids, i.e., abundant lipids that allow the membrane to be a bilayer, and rare lipids, which act as second messengers or as a flags of subcellular compartments (Di Paolo and De Camilli, 2006). PI(3,4,5)P3, a phosphoinositide with three phosphate groups on its polar head, is an example of a lipid messenger, acting as a compass for cell polarity (Servant et al., 2000). PI(3)P, with one phosphate group on its polar head group is an example of a flag lipid, being present exclusively on some organelles (endosomes) (Burd and Emr, 1998).
The most abundant phospholipids are phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylserine (PS). PC and PE are zwitterionic since their polar heads contain one negative and one positive charge, whereas PS is anionic since its polar head contains two negative charges and one positive charge (Holthuis and Levine, 2005).
Sphingolipids are deceptively similar to phospholipids as they also contain two acyl chains and one polar head group. However, subtle difference in the chemistry of sphingolipids compared to glycerophospholipids (longer acyl chains and more possibilities of hydrogen bounds) and the fact that complex sugar groups decorate the polar head make their physicochemical properties different.
Sphingolipids are abundant on the external face of the cell plasma membrane and play essential roles in cell protection and cell-cell communication (Holthuis and Levine, 2005; Simons and Sampaio, 2011).
Sterols are very different from both sphingolipids and phospholipids being made by a rigid carbon ring structure decorated, on the one hand, by a minimal polar group (a hydroxyl group) and, on the other hand, by a short carbon chain. A high concentration of cholesterol helps membranes to be impermeable to small molecules including water. However, the effects of cholesterol are complex and depend on the nature of the neighboring lipids.
Overall, the identity of each membrane-bound organelle results from the presence of unique proteins and of rare lipids, from the relative amounts of bulk lipids, and from some physical features such as shape or interaction with a protein matrix (e.g., the actin cytoskeleton underneath the plasma membrane).
In the case of synaptic vesicles that we have used before as an example, their noticeable features are the presence of key proteins involved in neurotransmitter uptake and release, the abundance of polyunsaturated phospholipids and cholesterol (40 mol% of all lipids), their extreme curvature (radius 20 nm), and their embedding in a dense protein matrix (Takamori et al., 2006). Endocytic vesicles are also very dynamic organelles whose formation and consumption correlate with abrupt changes in specific phosphoinositides species (Posor et al., 2013).
Most organelles exchange vesicles or other membrane- bound intermediates, a process referred as to membrane traffic. Consequently, the physicochemical features of many organelles vary according to their position along major trafficking routes. Thus, the endoplasmic reticulum (ER) and the plasma membrane define two extreme cases in term of lipid composition, whereas organelles such as the Golgi apparatus or endosomes display intermediate traits.
In this respect, two parameters define a first layer of organelle identity: membrane electrostatics, which depends on the abundance of negatively charged lipids (PS and phosphoinositides); and lipid packing, which depends on the presence of 'kinks' in fatty acid chains and on cholesterol levels (Bigay and Antonny, 2012; Holthuis and Levine, 2005).
Date added: 2024-06-13; views: 494;
