Mechanisms that Modifies the Bulk Lipid Composition of Organelles
Several mechanisms contribute to the nonrandom distribution of negatively charged lipids in the cell. First PI-kinases and PI-phosphatases are localized to well-defined organelles (Di Paolo and De Camilli, 2006). Second, PS is synthesized at the ER but is mostly present on the luminal side of this organelle and as such is inaccessible to cytosolic proteins (Fairn et al., 2011).
At the Golgi, transmembrane ATPases or 'flippases' promote the flip-flop of PS from the luminal to the cytosolic leaflet (Zhou and Graham, 2009), thereby making it available for the targeting of cytosolic proteins containing positively charged sequences.
How the cell gradually remodels the acyl chains of its glycerophospholipids, a process referred as to the Land's cycle, has remained mysterious for decades. Lipid remodeling involves the sequential action of phospholipases and acyl- transferases to replace the esterified acyl chains on the phospholipid glycerol backbone; for example, to convert a C18:1-C18:1 into a C16:0-C18:1 lipid.
The recent cloning of many acyltransferases and the realization that some members reside at the Golgi suggest that this organelle is a privileged region for changing the hydrophobic matrix properties (Shindou et al., 2009).
The activities of lipid flippases, acyltransfereases, and other lipid-modifying enzymes likely contribute to the large changes in membrane electrostatics, lipid packing, membrane asymmetry, and membrane thickness that occur at the Golgi, which defines the frontier of two main membrane territories (pre- and post-Golgi membranes). Of note, this frontier also acts as a filter to sort membrane proteins according to the physicochemical properties of their transmembrane domains (Sharpe et al., 2010).
Other mechanisms are being disclosed that maintain different lipid compositions between organelles. Cytosolic lipid transfer proteins extract one lipid from a donor membrane, convey it in the cytosol, and then deliver it to an acceptor membrane.
Thus, ceramide transport protein extracts ceramide from the ER and delivers it at the Golgi where ceramide is then transformed into sphingolipids (Hanada et al., 2003). Whether such lipid transfer activities are fast and specific enough to explain the distribution of some lipid species is a longstanding question. Recent studies suggest that two mechanisms might improve the efficiency of lipid transfer reactions (Kim et al., 2013; Mesmin et al., 2013).
First, some lipid transfer proteins are equipped with domains that bridge two membranes, leading to the formation of contact sites. Membrane contact sites allow the connection of almost any organelle to the ER, where most lipid building blocks are synthesized, and have a typical width of few tens of nanometers. Within this short distance, many lipid transfer reactions might occur in a coordinated manner.
Second, some lipid transfer reactions are coupled: the same protein can transfer one lipid in one direction and another lipid in the opposite direction. As such, phosphoinositide gradients might provide the energy for the fast transfer of lipids such as cholesterol of vitamin E (de Saint-Jean et al., 2011; Kim et al., 2013; Kono et al., 2013; Mesmin et al., 2013).
Membrane Curvature
Pure lipid bilayers are poorly extensible as their area can extend by about 5% before rupture. Because the surface of the plasma membrane is much larger than what would be required to encapsulate the cell, the cell responds to stretching through other mechanisms such as rupture of plasma mem- brane-cytoskeleton interactions or takes advantage small invaginations, the caveolae, which act as surface reservoirs (Raucher et al., 2000; Sinha et al., 2011).
In contrast, lipid bilayers can be highly bent (Rawicz et al., 2000). In the cell, membrane bending results from the actions of numerous protein machineries (McMahon and Gallop, 2005). The synaptic vesicle (radius 20 nm) is an example of a highly curved membrane that is formed by specialized membrane bending proteins.
The most complex organelle in term of morphology is the ER, which forms a continuous membrane network that emerges from the nuclear envelope and spreads throughout the entire cytoplasm. Many types of membrane geometries are met in this compartment (Shibata et al., 2009).
This includes not only long tubules and membrane sheets, but also small spherical vesicles, which transport cargoes toward the Golgi, nuclear pores with a torus-like shape, through which exchanges between the cytosol and the nucleus occur, and flat membrane contact sites with the plasma membrane.
The reticulons and the DP1/Yop1p proteins are transmembrane proteins that contribute to the formation of ER tubules and sheets (Figure 3(a)). These proteins display hairpin-like transmembrane segments that are not long enough to completely cross the bilayer. These hairpins are believed to occupy more volume in the cytosolic leaflet of the ER than its luminal leaflet, thereby leading to membrane deformation. Membrane curvature induced by asymmetric protein insertions in the bilayer matrix is referred as to the wedge mechanism.
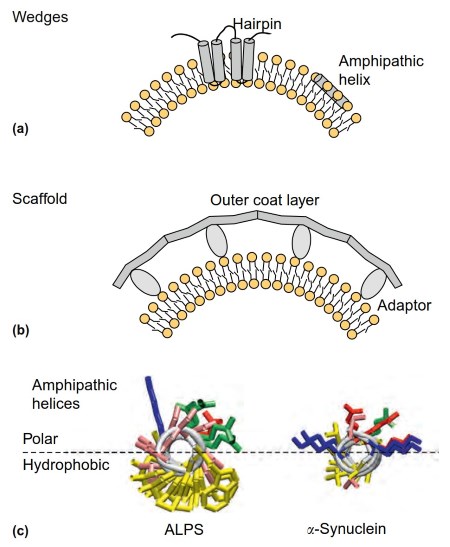
Figure 3. Shaping biological membranes. (a) Membrane deformation by wedges. Reticulon hairpins cross the membrane of the ER in an asymmetric manner, occupying more volume in the cytosolic leaflet than in the luminal one. Amphipathic helices are embedded in the interfacial membrane region, hence causing local deformations.
(b) Membrane deformation by scaffolds. Protein coats are typically made of two layers: one contacting directly the membrane and one polymerizing into a spherical scaffold. (c) Atomic models of two amphipathic helices (as viewed from the front).
These helices are adapted to different organelles. The helix on the left is an Amphipathic Lipid Packing Sensor: its polar face is made almost exclusively of small and uncharged residues (GLY, SER, THR; in pink), and its hydrophobic face contains bulky hydrophobic residues (LEU, PHE; in yellow).
This helix inserts into large lipid packing defects that form in the outer leaflet of small vesicles from the ER or the cis Golgi. The helix on the right is the N-terminal region of a-synuclein: its polar- face contains two wings of positively charged residues (LYS in blue) and one crest of negatively charged residues (ASP, GLU; in red).
Its nonpolar face contains very small hydrophobic residues (VAL, ALA; in yellow). This helix is adapted to vesicles containing negatively charged lipids and small lipid packing defects (e.g., endocytic vesicles arising from the plasma membrane)
The protein machineries involved in vesicle budding from the ER and in nuclear pore formation, the COPII coat and the nuclear pore complex (NPC), respectively, are based on a lattice-like arrangement of cytosolic proteins (Figure 3(b); McMahon and Gallop, 2005). NPC and coat complexes might have derived from the same ancestor (Schwartz, 2013). In both cases, the lattice is intrinsically curved and contacts the membrane indirectly via specific adaptors.
This mode of membrane deformation where a cytosolic protein or a protein complex imposes its intrinsic shape to the membrane without directly penetrating into the hydrophobic membrane matrix is referred as to the scaffolding mechanism.
The division between scaffolding and wedge mechanisms is instrumental to understand how simple proteins deform membranes (McMahon and Gallop, 2005). However, in many circumstances the wedge and scaffold mechanisms cooperate to shape biological membranes.
In the case of reticulon-like proteins, membrane deformation arises not only from the unbalanced chemistry of the hairpin segments but also from the ability of these proteins to oligomerize into arc-like structures. In the case of the COPII coat, the membrane deforming activity of the lattice is assisted by the membrane insertion of amphipathic helices from other proteins (Lee et al., 2005).
Amphipathic helices do not cross membrane bilayers but lie at the interface between the polar and nonpolar membrane region. Such shallow mode of membrane insertion is reversible and can be controlled by conformational changes (e.g., the GDP/GTP switch of small G Proteins).
As we leave the ER, cross the Golgi apparatus and reach the plasma membrane, we meet other membrane deforming machineries. The most well known are the COPI coat and the clathrin-AP adaptor coats, which share with COPII the same basic architecture: a lattice-like spherical outer layer and an inner layer made of cargo adaptors and amphipathic wedges. However, each coat is adapted to the membrane on which it acts.
The COPII coat is attached to the ER via the insertion of bulky hydrophobic amino acids of the small G protein Sar1 in the bilayer (Lee et al., 2005), whereas the clathrin coat contacts the plasma membrane via adaptors, which recognize the polar head of the phosphoinositide PIP2 and other anionic lipids (Jackson et al., 2010).
The predominate use of either hydrophobic or electrostatic interactions is also illustrated in Figure 3, which compares the chemistry of two amphipathic helices that adsorb on small vesicles budding from either early membranes (thus characterized by large lipid packing defects) or late membranes (thus containing anionic lipids). This comparison is suggestive of a molecular 'camouflage' where each helix is chemically adapted to the membrane interface in which it inserts (Pranke et al., 2011).
Many mechanisms of membrane deformation remain to be analyzed. The entry of some pathogens into the cell is suggestive of primitive modes of membrane deformations that bypass the requirement for specialized cytosolic machineries (Romer et al., 2007). Other interesting examples are the formation of cup-shape membrane structures during autophagy or the deformations induced by endosomal sorting complexes, which occur in the opposite direction as budding by protein coats.
Even in the case of protein coats for which spectacular advances have been made, key issues remain; notably how these well-defined protein assemblies cope with extremely crowded membrane surfaces or huge protein cargoes (Copic et al., 2012).
Date added: 2024-06-13; views: 478;
