Composition of Seawater. Chemical Properties of Water
Probably every element that occurs on earth is dissolved in seawater, the dilute solution filling the basins of the world ocean. Although the study of seawater is far from complete, we know that it is composed primarily of a dozen or so elements. The remaining elements are present in only small quantities. Water is certainly the most abundant component; it makes up about 96.5 percent of the weight of seawater.
Chemical Properties of Water. Much of the nature of our planet is determined by the properties of water. Elsewhere in this text the influence of water's unique physical properties is demonstrated. In this section the solvent properties of water are of primary interest. The solubility of substances, especially ionic compounds, those compounds yielding ions upon dissociation in solution (see the Appendix), is usually much higher in water than in other solvents.
Water is able to dissolve more substances than most known liquids. The remarkable solvent properties of water arise from the atomic structure assumed by liquid H20. Each oxygen atom is associated with two hydrogen atoms in an array as shown in Fig. 4-1A.
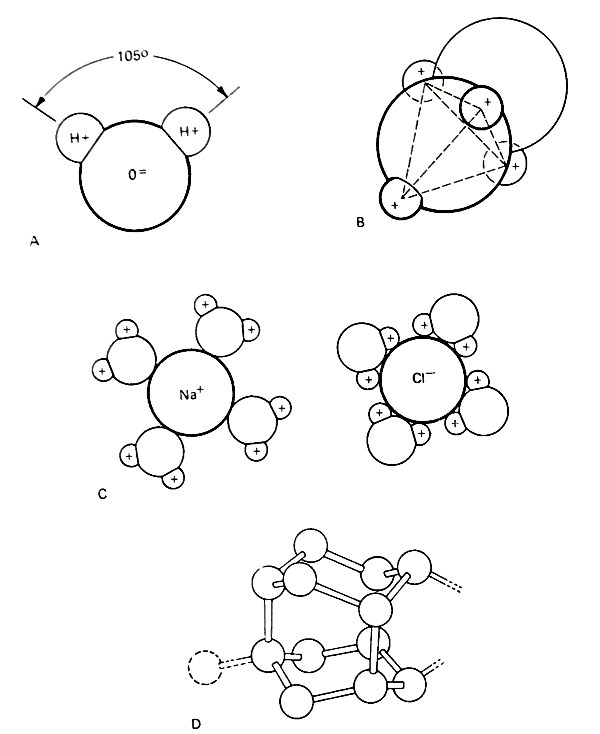
Figure 4-1 Various configurations of the water molecule. (A) The dipole arrangement of the hydrogen atoms in association with the oxygen atom; (B) The interaction of two water molecules showing the tetrahedral array of hydrogen atoms; (C) The hydration of sodium and chloride ions; and (D) The water tetrahedra bonded into hexagonal rings by hydrogen bonds shown as stick-like links. (After R. A. Home, Marine Chemistry, 1969, Courtesy of Wiley Interscience)
This is the form of water molecules in the gas state. Water vapor consists of individual H20 molecules. The two tightly bound hydrogen atoms lie on one side of the center of the H20 group and produce a net concentration of positive charge on that side. The side away from the two hydrogen atoms has a net negative charge. The uneven distribution of charge or polarization causes each H20 group to act as an electric dipole.
Because of this dipole configuration water molecules tend to interact strongly with each other as well as with other ions. The interaction between water molecules is such that a tetrahedral array of hydrogen atoms surrounds each oxygen atom (Fig. 4-1B).
Two of the hydrogen atoms are bound more loosely to the central oxygen atom but are bonded strongly to an adjacent oxygen atom. This structure is evident in ice where the water molecules are arranged in layers of hexagonal rings (see Fig. 4-1D). The electrical bonding of water molecules causes water to have an especially high latent heat of vaporization— the heat required to separate molecules when converting liquid to gas.
The unusual properties that water exhibits must be explained by the structure Of liquid water. Many theories have been postulated to explain the structure and unusual properties of liquid water. The theories attempt to explain the observed properties of water and aqueous solutions; all are successful to a large degree but all have flaws, and our understanding of water is as yet not total.
One group of theories holds that liquid water is a mixture of several forms of water such as free molecules, molecules bound in arrangements resembling that in ice or other regular arrangements, and molecules in random arrangements. Another group of theories portrays water as a continuum, in which the arrangement of water molecules changes with temperature and pressure.
A major shortcoming of such theories is that they inadequately explain the fluidity of water (water is too fluid) and the difficulty with which water freezes (nuclei for freezing do not appear to exist in liquid water). These shortcomings are overcome in a theory that describes liquid water as a mixture of individual water molecules and clusters of bound water molecules.
The clusters exist for 10-10 to 10-11 seconds before they disaggregate. An individual water molecule spends part of the time as a free molecule bound to other molecules by dipole attraction and by the electrostatic attraction between protons m one molecule and the electrons in another.
The rest of the time it is bound in a cluster by rather strong hydrogen bonds. The clusters have an open structure and are less dense than an equal volume of unstructured water molecules. Consequently, an increase in pressure tends to prevent cluster formation, thus increasing the density of water.
The fluidity of water first increases with pressure and then decreases as pressures increase above 300 to 1,000 atmospheres. It appears that initially the destruction of clusters leaves space in which individual water molecules may move more freely; at pressures where clusters can no longer form, an increase in pressure tends to crowd individual water molecules such that they interfere with each other's freedom of movement.
As the temperature increases from 0° to 100°C, a small number of large clusters is replaced by a larger number of smaller clusters; nonetheless, the number of water molecules bound in clusters decreases with a rise in temperature. The existence of a water density maximum at 4°C has been explained as cluster destruction causing increased density from 0° to 4°C. Above 4°C, thermal expansion caused by increased thermal motion of individual water molecules exceeds the decrease caused by cluster destruction.
At pressures that exist in the ocean (1 to 1,100 atmospheres), substantial amounts of water molecule clusters persist over the entire liquid range of 0° to 100'C. This is important in considering the effects of dissolved salts in seawater. Ionic compounds dissolve readily in water because water dipoles are attracted to and surround each charged ion with a sheath of oriented water molecules (Fig. 4-1C).
This effect, called hydration, prevents recombination of dissolved ions and accounts for the large solubility of ionic salts. Nonionic compounds are soluble in water not because they dissociate to form charged ions that can become hydrated, but because the charge distribution in these molecules is irregular and a sheath of water dipoles hydrates and is attracted to sites of high charge.
The electrical charges surrounding chemical species dissolved in water cause the formation of two zones in which the structure of water is altered (Fig. 4-2). Next to the dissolved solute, the water molecules are bound strongly to the extent that they are electrostricted by the charge of the solute and are oriented in a configuration in which they are relatively immobile.
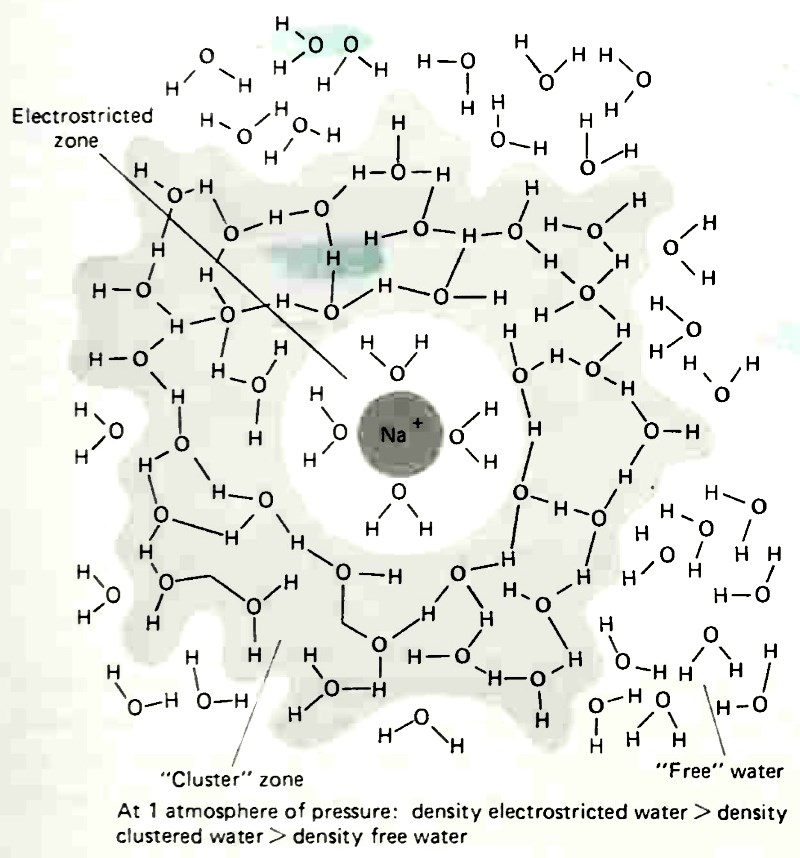
Figure 4-2. Two-dimensional representation of the total structure-enhanced hydration atmosphere of the sodium ion. (After R. A. Home, Surv. Prog. Chem., copyright 1968 by Academic Press, Inc.)
The water in this zone is considerably more dense and less fluid than ordinary water. Surrounding the electrostricted zone is another zone in which the charge of the solute is insufficient to produce electrostriction or orientation of water molecules but is strong enough to disrupt the formation of clusters of water molecules. The inner zone is one of enhanced water structure and it is thought to exist around all solutes. The outer zone is one of broken structure and increased fluidity. The varied behavior of various solutes in water is thought to be related to their ability to form a broken structure zone.
Date added: 2024-04-08; views: 804;
