Chemical Transport. Definitions. Fluxes in the Ocean
Many of the chemical properties of the world ocean are the result of the transport of dissolved and suspended materials from one region to another laterally and vertically. We can understand the distribution of chemical properties in the ocean if we know how the distribution is accomplished. Conversely, a mapping of the distribution of chemical properties elucidates transport mechanisms operating in the ocean. In this chapter we shall examine this relationship between chemical distributions and chemical transport mechanisms.
The chemistry of the ocean is characterized by mixing processes that cause materials introduced into the ocean from several sources to be distributed uniformly. On the other hand, regulatory processes tend to segregate materials and prevent ultimate homogenization of seawater. The interaction of these processes is quite complex, but we can gain an understanding of the chemistry of the ocean by systematically applying the concepts of chemical transport. Before we do, several definitions are necessary.
Definitions. The chemicals of the sea exist as distinct species, each having characteristic chemical properties. Ionic species of the major elements in seawater have been discussed; a list of the chemical speciation of other elements is presented in Table 5-1. It is common for an element to exist in several species. Chemical species are distributed among the aqueous, solid, and gaseous phases of the ocean. Fats and oils, being immiscible in water, constitute a minor phase in the ocean. Bubbles of air constitute the bulk of the gaseous phase in the ocean.


Table 5-1. Abundance and Speciation of Elements in Seawater
We can regard the ocean as a chemical system consisting of reservoirs of chemical substances connected by pathways along which those substances are transferred. The amount, or mass, of chemical substance in a reservoir is expressed as a concentration. Several conventions exist for concentration. Most describe the mass per unit volume or the relative number of molecules in a unit volume. Weight percentage is a useful concept as well. The transfer of material along pathways is described in terms of a flux: the amount (mass) transferred through a unit area in a unit time.
Fluxes in the Ocean. If the transfer is by means of fluid flow, it is termed advection. The advective flux of a liquid is the product of its density and its velocity of flow:
Mass flux of liquid = Density x Velocity
For most purposes of chemical oceanography, seawater density is expressed by the approximate value 1 g/cm3. The advective flux of a chemical species, A, dissolved in seawater is the product of the concentration of the species (written, by chemical convention, as [A]) and the velocity of flow of seawater:
Flux = [A] x Velocity
Solid particles are transported to the ocean as suspensions in rivers. Within the ocean, turbulent motion moves particles of sediment and organic debris. Ultimately, gravity carries an advective flux of particulate material to the sea floor. The particulate flux of a chemical species, B, is the product of the concentration of the species in the particular phase, [B], the concentration of particles in space, ф, and the velocity of the motion of the particles:
Flux = [В] x ф x Velocity
Where the concentration of a dissolved chemical species in the ocean is not uniform, transport by diffusion occurs. In diffusion, dissolved particles (ions or molecules) move from a region of high concentration to a region of lower concentration by virtue of their energy of motion. Their rate of travel is governed by the magnitude of their energy of motion (influenced strongly by temperature) and by the frequency with which they can collide with neighboring particles; this is characterized by a coefficient of diffusivity, D.
The diffusive flux is determined by (1) the diffusivity coefficient, (2) the difference between the concentrations of the chemical species, C, at the region of high concentration and the region of low concentration, and (3) the distance separating the regions. The diffusive flux is expressed as:
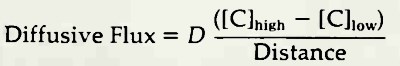
Two kinds of diffusive flux are found in the ocean. In the absence of turbulence, the diffusive flux follows the equation above, which is called Fick's first law of molecular diffusion, Fickian diffusion takes place slowly and is most effective over relatively short distances. If turbulence is present, the diffusive flux is minor compared to that of turbulent mixing Turbulent mixing of masses of water resembles a diffusive transport process, and the appropriate coefficient in the flux equation in such a case is a coefficient of eddy mixing. Mixing by turbulent eddies is quite rapid and takes place over distances of tens or hundreds of meters in the open ocean.
The flux of a gaseous species is diffusive; transport between the gaseous and aqueous phases resembles the Fickian equation except that an exchange coefficient analogous to diffusivity characterizes the process.
Date added: 2024-04-08; views: 621;
