Chemical Kinetics in Environmental Systems
Introduction. Thermodynamics is the study of energy and its transformations. Chemical thermodynamics describes the overall energy change that a system experiences upon a change in state (such as with an increase in temperature and pressure), which is independent of the pathway taken. Chemical kinetics, on the other hand, is the study of rates and mechanisms of reactions.
Kinetics focuses on the reaction pathways, including the time dependency of reactions and the detailed reaction sequences. There are some instances in which a reaction is reversible and fast enough that is can be regarded as essentially equilibrium controlled. However, many systems of environmental interest involve slow or irreversible reactions, cannot be considered at equilibrium and therefore require application of kinetic models.
This article is drawn heavily from P. Maurice’s 2009 book on Environmental Surfaces and Interfaces from the Nanoscale to the Global Scale. However, it has been substantially edited and updated. This topic is important to the overall Encyclopedia because many biogeochemical processes that occur in water and/or strongly affect water quality are kinetically influenced.
Many times, environmental scientists are interested in figuring out the rates and mechanisms of reactions of interest in aquatic systems. This article can be considered as a companion article to Some fundamentals of thermodynamics as applied to freshwater chemistry, also published in the Encyclopedia.
A Brief Introduction to Kinetics. Whereas thermodynamics describes the overall energetics of a reaction and allows prediction of which direction a reaction will proceed spontaneously, kinetics describes the rates and detailed mechanisms of reactions. Many systems at and near the Earth’s surface show strong kinetic influences, especially when processes are biologically mediated.
Homogeneous Versus Heterogeneous Reactions. In kinetics, it is important to consider whether a reaction is homogeneous, in which only one phase (such as gas or liquid) is involved, versus heterogeneous, in which different phases (such as a solid mineral in liquid water) are involved. Heterogeneous reactions, such as dissolution of an oxide mineral in water, are often either reversible but slow, or even irreversible, thus requiring kinetic approaches.
Overall Versus Elementary Reactions. Chemical reactions are written in terms of reactants and products. Most of the reactions we are used to seeing in introductory chemical texts are actually overall reactions, such as: A + B → AB (1)
This equation shows that components A and B reacted to form AB; however, this overall reaction tells us nothing about the detailed reaction sequence.
An example of an equation for an overall reaction is for dissolution of the clay mineral kaolinite Al2Si2O5(OH)4 to form gibbsite Al(OH)3;
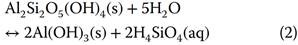
This overall equation only describes the initial reactants and the reaction products. In reality, reactions may take several or many steps, each of which is known as an elementary reaction. For example:


where the k values are the rates of the different elementary reactions in the directions written. This reaction sequence would result in the overall reaction (1), but Eq. (1) does not provide any hint of the detailed sequence of elementary reactions involved. An elementary reaction is assumed to occur as a single step and to pass through a single transition state (see below).
It is impossible to tell from an equation for an overall reaction how many steps are involved. Most reactions occur by a sequence of elementary reactions, each of which generally involves only one or two molecules. An elementary reaction might be, for example:
H + Br2 → HBr + Br (5)
This equation for an elementary reaction signifies that a particular H atom attacks a particular Br2 molecule to produce a molecule of HBr and a Br atom.
Date added: 2023-10-03; views: 784;
