Highlight: Diverse Behavior of the Indicator Phenolphthalein
We now discuss the behavior of the common acid-base indicator phenolphthalein in somewhat greater detail than given above for generic indicators. Most readers are likely already aware that solutions containing phenolphthalein are colorless over a range of circum-neutral and acidic pH values, but have a pink (or magenta) color at pH values > = 9.
However, some readers may not be aware that this indicator undergoes further changes in ionization state (and coloration) at pH values above and below the pH range of its typical application. In this sub-section, we discuss the behavior of this indicator over the broad range of pH from less than zero to greater than 12. This indicator undergoes four transitions between ionization states (not just one, as in the simplified generic discussion above), and is accordingly distributed across five unique ionization structures as a function of pH. We refer to these structures (1-5 in Figure 10) as H3PP+, H2PP, HPP-, PP2- and PP(OH)3-.1
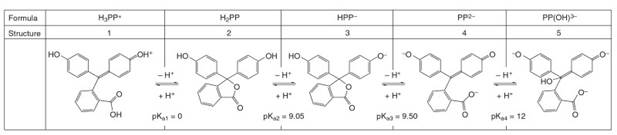
Figure 10. pH – dependent ionization structures of the indicator phenolphthalein (PP)
The pKa values and the structures, which are supported by infrared spectroscopic evidence, are drawn from Tamura et al.
We begin our discussion with structure 1. This fully protonated (H3PP+) form of the indicator is dominant in solutions with pH < 0. The central carbon of this structure atom has sp2 hybridization, which enables two of the three peripheral aromatic rings to be connected in one extended electronic conjugation network. The HOMO/LUMO energy gap is relatively small for this structure, and its electronic excitation is achieved with visible photons. This structure thus imparts a vivid red/orange color to the (very low pH) solutions in which it is dominant. This form, although interesting, lies outside the normal range phenolphthalein’s application.
We consider next the ionization structure which is produced by deprotonation of 1. Solutions (pH range 0=9), in which the neutral form 2 (H2PP) is dominant, are colorless. In form 2, the hybridization of the molecule’s central carbon atom is converted from sp2 to sp3, which disrupts the conjugation network and electronically isolates the three peripheral aromatic groups. Small, electronically isolated benzene derivatives such as these have relatively short conjugation lengths, and consequently, relatively large HOMO/LUMO energy gaps. Electronic excitation of any of these isolated groups requires high energy UV photons, thus resulting in a colorless solution.
Deprotonation of structure 2 (pKa« 9.05) generates structure 3. In structure 3 the central carbon atom is still sp3 hybridized, which would suggest this form should also be colorless. However, it is noted that structure 3 can exist in a rearranged form (3', not shown) in which the central carbon atom is sp2 hybridized [6]. At the very close and slightly higher pH of 9.05, structure 3 is depro- tonated to generate structure 4, which fully hydrolyzes the lactone ring of the central carbon and reverts this carbon’s hybridization state to sp2.
Solutions containing mixtures of structures 3, 3', and 4 are pink (magenta) in color and have similar visible absorption spectra. Advanced characterization techniques, such as infrared spectroscopy, are needed to observe this diversity of ionization state structures. In typical applications of the indicator, it is assumed that the indicator has a single form above pH = 9. Regardless of the assumptions made about the ionization state behavior, the color change (colorless to pink) that occurs at about pH 9 is frequently used in practicality to locate the endpoints in the titrations of acids by strong bases. At even higher values of pH, phenolphthalein has one more change in ionization state.
At solution pH values approaching 12, structure 4 is then converted into structure 5 via nucleophilic attack by OH- ion at the position of the central sp2 carbon atom in structure 4. This reaction, which converts the indicator into the PP(OH)3- form (Figure 10 structure 5), follows a Michael addition mechanism, and changes the central carbon’s hybridization back to sp3.
This transition again disrupts the ion’s electronic conjugation, and converts the solution once again to colorless. The pH-dependent colors of other acid-base indicators can similarly be rationalized in terms of the differences in structures and electronic π-conjugation lengths in their various ionization state structures.
Date added: 2023-10-03; views: 690;
