Metal Speciation Diagrams
Another common application of thermodynamics as related to water chemistry is calculation of the distributions of metal species at equilibrium, resulting in a plot of metal species concentrations versus pH. Which species are present for a given metal such as Cu, Zn, Co, Cd or Pb can greatly impact phenomena such as metal adsorption to mineral surfaces and mineral solubility.
Determining the concentrations of metal species at equilibrium in a given solution requires a carefully reviewed and consistent thermodynamic compilation of stability constants and hand calculation or use of a thermodynamic computer model such as PHREEQC or one of the other models mentioned below.
An example of a speciation diagram for Cd(II) in the presence of the organic ligand desferrioxamine B (DFO-B) is shown in Figure 4.
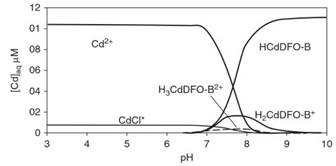
Figure 4. Speciation diagram for Cd (11.1 pM) in the presence of the organic microbial ligand DFO-B. In 0.1 M NaClO4 background electrolyte solution, at 22 °C. After Hepinstall et al.
In this diagram, the concentrations of the various Cd species are given on the y-axis (generally in molL-1) and pH is on the x-axis. At pH < 7, the predominant Cd species is Cd2+, with a small amount of CdCl+. Other species present are at concentrations too low to see clearly on the plot. At pH > 7, Cd-DFO-B complexes HCdDFO-B, H2CdDFO-B+ and H3CdDFO-B2+ become important. The total concentrations of chemical constituents, the temperature, and the ionic strength play important roles in the construction of speciation diagrams.
Thermodynamic Models of Water Chemistry. Garrels and Thompson developed a method for modeling complex aqueous geochemical equilibria using calculations performed by hand. Over the decades since then, a wide variety of computer software programs have been developed for modeling water chemistry. While most focus on thermodynamic modeling, many now also contain at least some kinetic calculations.
The models can be used for applications such as acid-base reactions, aqueous complexation and species distributions, dissolution and precipitation of mineral phases, redox reactions, and, in some cases, sorption to mineral and/or microbial surfaces. Some of the models that are currently in widespread usage include (amongst others) PHREEQC, Visual Minteq, the Geochemist’s Workbench, and WHAM.
While these models can provide rapid and widely useful information on a water sample and an aqueous environment, it is important for the user to assure that the data entered into the model have gone through careful quality control, that the model is operating correctly with a valid database for the system at hand, and that the results are interpreted properly.
For example, even though a model shows that a stream water sample is undersaturated with respect to a given mineral phase, the user should not presume that such a mineral phase is dissolving in the stream watershed, as it might not even be present in the local geologic milieu.
Date added: 2023-10-03; views: 670;
