Transport Versus Surface Control of Mineral Growth and Dissolution Rates
Three of the most important reactions that occur at the mineral-water interface are inner-sphere adsorption, mineral growth, and mineral dissolution. These three processes include the following steps: (i) transport of reactant ions or molecules from the solution to the surface, (ii) surface diffusion to a reactive site, (iii) attachment to a site on the surface and in the case of mineral dissolution, and (iv) detachment back to the solution.
Other processes such as solvation and desolvation are also important, in many instances. Since (i)-(iii), (iv) occur sequentially, it is the slowest step that is rate determining. Because of this, the rates of mineral growth and dissolution are often considered in terms of transport versus surface control.
The rates of growth and dissolution of various minerals each may be controlled by transport processes, surface processes, or an intermediate case of both. Let us consider mineral growth in some detail, with dissolution being essentially (although often not exactly) the converse. Transport-controlled crystal growth in aqueous solutions is generally thought to be sensitive to flow (or stir) rate because this controls the rate of transport (by diffusion and advection) of reactants to the surface.
This sensitivity to flow rate is only up to the point when flow rate is so fast that transport no longer limits the supply of reactants. Surface-controlled crystal growth is thought to be insensitive to flow rate (or stir rate) because the surface process is slow relative to transport. Surface-controlled growth is generally slower than transport-controlled growth and has greater temperature sensitivity associated with higher activation energy. Surface-controlled growth can be limited by surface diffusion or by attachment, depending upon the particular conditions.
For surface-controlled growth, various imperfections such as steps and kinks on a surface can be important because sites associated with the imperfections have different coordination environments and hence different energies. Therefore, different rates of reaction may be associated with different surface features. If growth is occurring simultaneously at several different sites, then the site with the fastest growth rate generally determines the overall rate.
Figure 3 illustrates the concentration gradient of reactants that occurs around a growing crystal under static conditions and transport- versus surface-control of rate. In transport-controlled growth, reactants become depleted from the vicinity of the surface. However, such a gradient in reactant concentration does not occur in surface-controlled growth. As stir rate is increased, the gradient decreases and eventually disappears. An intermediate case has only a slight depletion near the surface.
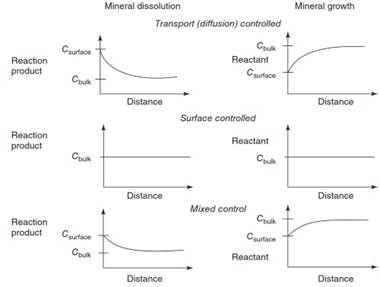
Figure 4. Time to obtain equilibrium for some processes in porous media. Substantially revised from
Mineral dissolution also can be transport- or surface- controlled. For transport-controlled dissolution, the rate-determining step is transport of the reaction product away from the surface. In transport-controlled dissolution systems, reaction products build up near the surface. Dissolution rate is then limited by the rate at which the dissolution products are transported (by diffusion and advection) away from the surface. The reactant also can be at lower concentration near the surface than in the bulk solution, and this can also affect the dissolution rate.
For surface-controlled dissolution, the rate-determining step is a reaction at the surface, probably most often the detachment from the surface of a metal or metal-ligand activated complex. In this case, the concentrations of reaction products do not build up near the surface. At steady state dissolution, the dissolution kinetics follows the rate law (rate of dissolution Rd in mol s-1):

where C is the concentration of product, t is time, k is the rate constant (in mol m-2 s-1) and A is the surface area of the mineral (in m2). As for mineral growth, sometimes dissolution may be intermediate, with both controls contributing to the overall dissolution process.
When dissolution rate is surface reaction controlled, mineral surfaces tend to form structures such as pits and ledges because the dissolution process is fastest at certain high-energy sites on the surface. However, when dissolution is transport controlled, the mineral surface tends to be more rounded without such distinct dissolution features. Minerals that dissolve faster, such as halite (NaCl) and gypsum (CaSO4-2H2O), tend to do so by transport-controlled mechanisms.
Minerals that dissolve more slowly such as the feldspars and hematite tend to do so by surface-controlled mechanisms. One of the most abundant and geochemically significant minerals, calcite (CaCO3), can dissolve by either surface or transport control. Factors such as solution pH, saturation state, temperature, presence of impurities, and particle size of a mineral can influence whether a mineral dissolves by surface-, transport- or combined or “complex" mechanisms in any particular setting.
Transport- and surface-limited growth and dissolution can be considered to be end-member situations, with many minerals growing or dissolving at rates that are a more complex function of both transport and surface processes.
Date added: 2023-10-03; views: 677;
