Defenses Against Oxidation. Enzymatic Defenses. Nonenzymatic Control of Oxidation
If oxygen generates free radicals in cells and free radicals are so damaging to cellular constituents, how can we survive as long as we do in our oxygen-containing atmosphere? How can we protect our foods against deterioration due to oxidation? Part of the answer is that our bodies and other biological systems have evolved defense mechanisms to remove or scavenge free radicals and to repair or excrete molecules damaged by free radical attack. It appears, however, that defenses intrinsic to body cells are not sufficient to adequately protect against excessive oxidation and we must supplement those defenses with dietary antioxidants.
We should not forget that even though peroxides and free radicals can cause serious damage to the body, they also play beneficial roles. For example, phagocytes (cells in the immune system that engulf and kill invading bacteria and viruses) purposefully generate hydrogen peroxide and superoxide, presumably to aid in the killing of the invading organism. There is also evidence that superoxide may play a role in intercellular signaling and growth regulation.
So there seems to be a delicate balance. Peroxides and free radicals are beneficial at low concentrations in the right places but may be toxic when concentrations are too high or when they are generated where they can cause harm. When this balance is tipped toward excess oxidation, cells and organisms are said to be under oxidative stress.
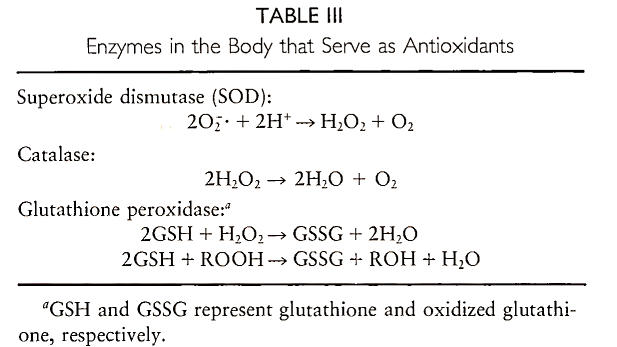
A. Enzymatic Defenses. Cells contains enzymes that can destroy free radicals and peroxides. These include superoxide dismutase, which converts superoxide to hydrogen peroxide, catalase, which converts hydrogen peroxide to water and molecular oxygen, and glutathione peroxidase, which catalyzes the decomposition of both hydrogen peroxide and lipid hydroperoxides (Table III). Glutathione is a tripeptide composed of glutamine, cysteine, and glycine residues. It is an important antioxidant in cells. Glutathione peroxidase requires selenium for activity. Thus, dietary selenium deficiency can impair the body’s ability to defend against the damaging effects of peroxides.
B. Nonenzymatic Control of Oxidation. Apparently, the enzymatic defense systems just mentioned are not capable of completely protecting against damage by free radicals. Therefore, other mechanisms for removing free radicals are needed. This is where antioxidants come in. Antioxidants are chemical species that can prevent, slow, or eliminate oxidation. Since much of the damaging oxidation in cells occurs when free radicals attack various cell components, most of the antioxidants act by either scavenging free radicals or preventing their formation in the first place. Antioxidants in foods include α-tocopherol (vitamin E), ascorbic acid (vitamin C), B-carotene and other carotenoids, BHA, BHT, plant polyphenolics, and various metal chelating agents (EDTA, citric acid, phytic acid).
It should be clear from the preceding sections that oxidation of organic molecules requires oxygen and free radicals. In addition, transition metal ions may initiate and accelerate lipid peroxidation by catalyzing the formation of new free radicals. Light may initiate peroxidation, transforming triplet oxygen into singlet oxygen and thereby allowing the formation of lipid hydroperoxides, which can decompose to form free radicals. How then might lipid peroxidation in foods and/or our bodies be controlled? Several strategies are available.
1. Elimination of Oxygen. Oxygen is required for many of the oxidation reactions in foods and the body. Therefore elimination of oxygen should prevent peroxidation. This strategy is used in many food products. It is accomplished by vacuum packaging or by flushing away oxygen with nitrogen gas and sealing the food in packages that are impermeable to oxygen. Obviously, this strategy cannot be used for controlling oxidation in the body because oxygen is required for energy metabolism.
2. Scavenging of Free Radicals. Given that many of the oxidative reactions that occur in foods and the body involve a free radical mechanism, it stands to reason that removal of free radicals would prevent or slow oxidation. In fact many of the most effective antioxidants in foods and the body are free radical scavengers. To understand how free radical scavengers work, we will take a-tocopherol (vitamin E) as an example (Fig. 3).
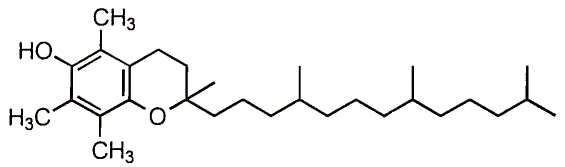
FIGURE 3. The structure of a-tocopherol showing the hydroxylated aromatic ring and the long hydrocarbon tail. a-Tocopherol is the most active form of vitamin E
Vitamin E is a phenolic compound, that is, it contains an aromatic ring with an attached hydroxyl group. The hydrogen in the hydroxyl group of a phenolic compound is relatively easily abstracted. Thus phenolic compounds will donate a hydrogen atom to free radicals converting them to nonradicals:
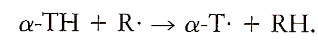
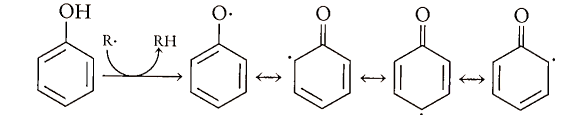
We still have a free radical, a-T However, phenolic free radicals are relatively stable and are incapable of abstracting a hydrogen from another unsaturated fatty acid. Thus, the chain is broken and lipid peroxidation is slowed. Phenolic radicals are stable because of resonance delocalization around the aromatic ring:
3. Chelation of Metal Ions. Since transition metal ions catalyze the formation of free radicals, we might expect that removal of metal ions would reduce oxidation. However, it is not possible or practical to remove them from foods or the body. Iron and copper are both essential nutrients, moreover, iron deficiency is a widespread nutritional problem. Therefore, even if it were possible to remove these metals from foods, it would be unwise to do so. In fact, iron is added to many foods to ensure adequate intakes by individuals in the population.
Fortunately, chelation of metal ions by chelating agents often reduces their effect on oxidation. Recall that metal ions can form complexes (coordination compounds) by attaching to various ligands through coordinate covalent bonds:
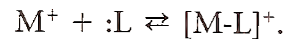
In this reaction, the metal ion is acting as a Lewis acid (an electron pair acceptor) and the ligand is acting as a Lewis base (an electron pair donor). Many ligands are organic molecules that contain atoms (usually oxygen or nitrogen) capable of donating a pair of electrons to form the coordinate covalent bond with metal ions. Often these molecules contain more than one donor atom, allowing the formation of multiple coordinate bonds between the metal ion and the ligand. When this is the case, the complex that forms is called a chelate. Structures of EDTA, a strong chelating agent capable of forming six bonds with metal ions, and iron-EDTA chelate are shown in Fig. 4.
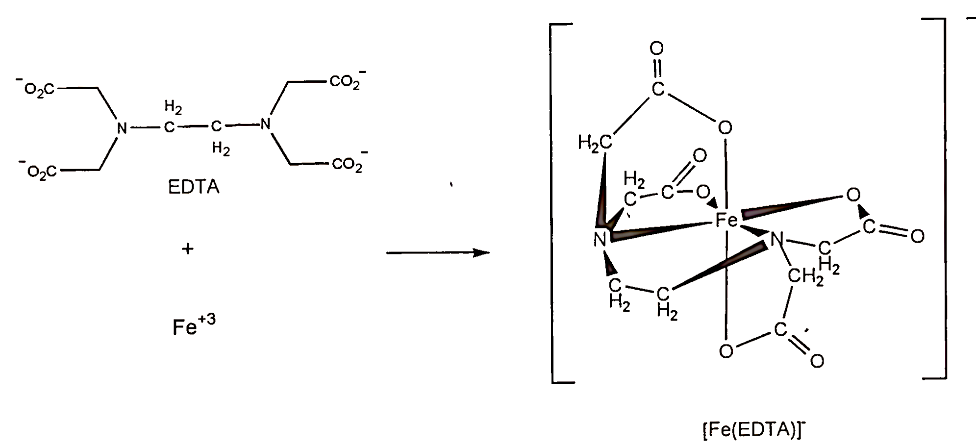
FIGURE 4. Ethylenediaminetetraacetate (EDTA) and its chelate with Fe3+
When metals combine with ligands to form chelates, their electronic structures are altered and this affects their ability to participate in other reactions. As mentioned earlier, chelated metals may not catalyze oxidation reactions in the same way as free metal ions. Thus oxidation can often be controlled by adding a chelating agent to a food. The cells in plants and animals synthesize a variety of chelating agents that apparently function to prevent oxidation, including the proteins transferrin and ferritin. Transferrin is a protein that circulates in the blood serum. It binds iron extremely tightly and serves to transport iron from the intestine, where it is absorbed, to body cells, where it is needed. Ferritin is a protein that sequesters iron in storage sites. It is present in greater concentration in liver, bone marrow, and spleen, important storage sites for iron.
Date added: 2023-01-09; views: 673;
