Some Common Food Antioxidants
I. Radical Scavenging Antioxidants. a. Vitamin E. The term “vitamin E” refers to a family of compounds that contain a hydroxylated aromatic ring. These compounds are known as tocopherols and tocotrienols. The hydroxylated aromatic ring makes them members of the class of compounds known as phenols or phenolic compounds. The most active form of vitamin E in animals is rx-tocopherol (Fig. 3). Vitamin E is highly lipophilic and resides primarily in cell membranes and lipoprotein particles in the blood (e.g., low density lipoprotein and very low density lipoprotein).
Vitamin E protects against oxidative damage to polyunsaturated fatty acids by scavenging free radicals. In the process, a tocopherol radical is generated. As mentioned earlier, phenolic free radicals are relatively stable and will not abstract a hydrogen atom from another fatty acid, thereby breaking the chain reaction. Vitamin E may be regenerated from the tocopherol radical by the action of ascorbic acid (vitamin C).
Tocopherols and tocotrienols are widely distributed in plant foods. Vegetable oils, whole grain cereals, nuts, and green leafy vegetables are particularly good sources. The vitamin E content of animals foods is generally very low.
b. Vitamin C (Ascorbic Acid). Ascorbic acid is a water-soluble antioxidant and is also a free radical scavenger. It regenerates vitamin E from tocopherol radicals and can also scavenge other free radicals, including superoxide, peroxyl, thiyl, and hydroxyl radicals. In addition, ascorbic acid can quench singlet oxygen in aqueous solution. Some nutritionists and promoters of nutrient supplements advocate vitamin C intakes far in excess of the RDA.
The use of high-dose vitamin C supplements is controversial because of the possibility that vitamin C can act as a pro-oxidant under some conditions. Recall that ferrous iron (Fe2+) can catalyze the formation of hydroxyl radical from hydrogen peroxide and that ascorbic acid is capable of reducing ferric iron (Fe3+) to ferrous iron. In vitro experiments have clearly shown that under conditions where ferric iron and unsaturated fatty acids are present, ascorbic acid actually promotes lipid oxidation. Whether this also occurs in vivo is not known.
Citrus fruits, green peppers, cauliflower, broccoli, cabbage, and strawberries are particularly good sources of vitamin C.
c. Carotenoids. Carotenoids are plant pigments composed of isoprene units covalently linked together giving them multiple conjugated double bonds (Fig. 5). Colors of carotenoids range from yellow to orange to red. Although animals cannot synthesize them, some animal foods contain carotenoids because animals absorb, modify, and deposit dietary carotenoids in tissues. The yellow in egg yolk, for example, is due to carotenoids. Some carotenoids may be converted to retinol (vitamin A) in the body, thus these carotenoids have vitamin A activity. There are two main groups of carotenoids, the carotenes and the xanthophylls. The carotenes are hydrocarbons (they are composed of only carbon and hydrogen) and the xanthophylls contain oxygen in their structures. Carotenoids may function as free radical scavengers; they also quench singlet oxygen.
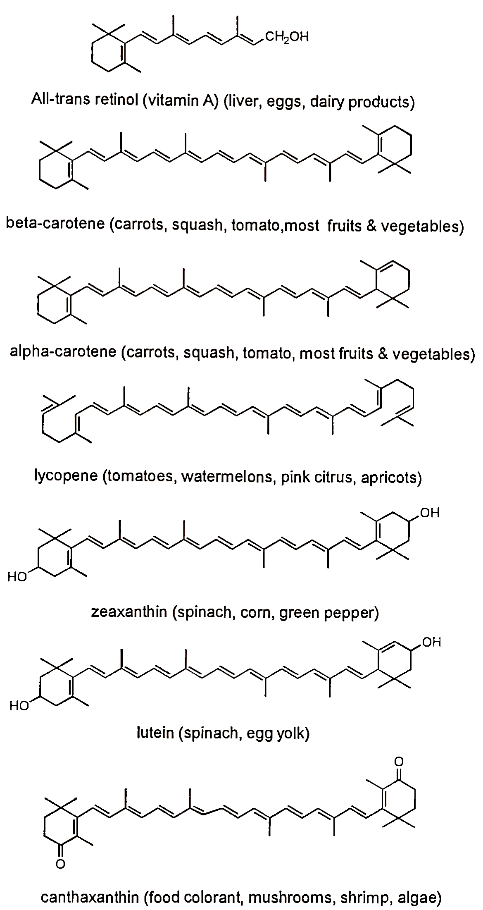
FIGURE 5. Structures and food sources of some selected carotenoids. Of the carotenoids listed, only retinol and a- and /3-carotene have vitamin A activity
Carotenoids with vitamin A activity are found in yellow and orange vegetables and fruits and in many dark green vegetables. Carrots, squash, sweet potatoes, spinach, broccoli, papayas, and apricots are good sources.
d. Plant Phenolic Compounds. In addition to vitamin E, plants contain many other phenolic compounds (Fig. 6). Many of these compounds contain aromatic rings with more than one hydroxyl group. Thus they are referred to as polyphenols or polyphenolic compounds. The general term “plant phenolics” includes compounds ranging from relatively low-molecular-weight phenolic acids such as caffeic acid to high-molecular-weight polymers known as tannins. Many plant phenolics are pigments. For example, the reds and blues in grapes, plums, cherries, and strawberries are due to the presence of a subclass of phenolic compounds called anthocyanins. Tea and coffee contain substantial concentrations of catechins and their derivatives.
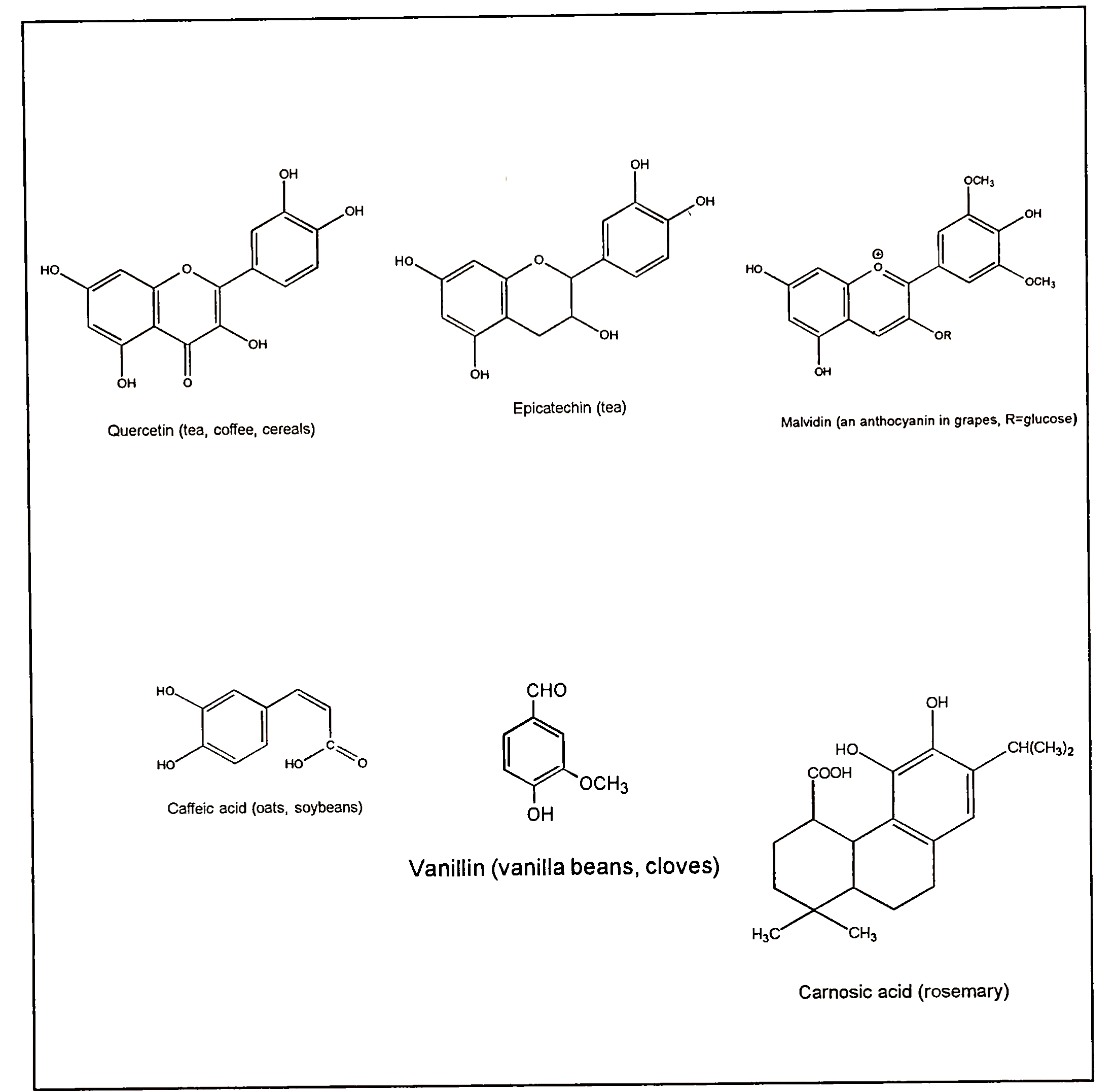
FIGURE 6. Selected examples of naturally occurring phenolic antioxidants (sources of the compounds are shown in parentheses)
Catechins may be converted to tannins by oxidative polymerization reactions, and tannins impart an astringent taste to foods. Many spices are also rich sources of phenolic compounds and are added to
foods to add flavor but also to prevent oxidation. As we would expect, based on their chemical structures, plant phenolics are antioxidants. They exert their antioxidant effects by scavenging free radicals, quenching singlet oxygen, and, in some cases, chelating metal ions.
e. Synthetic Antioxidants. Many foods that contain polyunsaturated fatty acids are susceptible to oxidation. Thus food scientists have developed a variety of compounds that can prevent or retard lipid oxidation in foods. The primary synthetic antioxidants used by the food industry are butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), tertiary-butyl hydroquinone (TBHQ), and short-chain esters of gallic acid (propyl gallate) (Fig. 7).
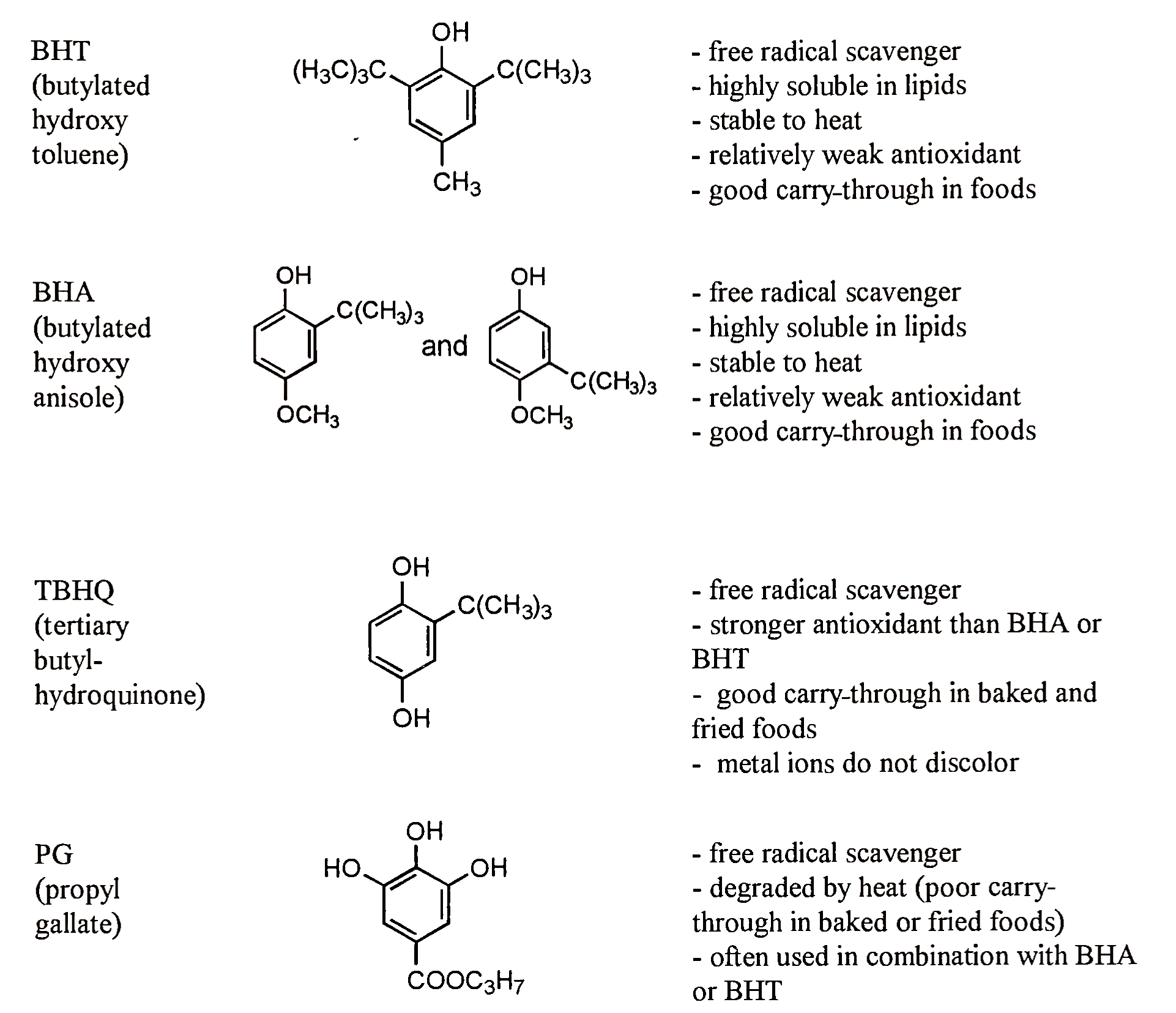
FIGURE 7. Synthetic antioxidants approved as food additives
Antioxidant activity and lipid solubility of these compounds are influenced by the number of hydroxyl groups on the aromatic ring and the number and size of substituted alkyl groups. When choosing which synthetic antioxidant to use, food manufacturers must determine what qualities the antioxidant must have in order to be effective. BFTT is highly nonpolar because the two tertiary butyl groups on the molecule make it very lipid soluble. However, the bulky tertiary butyl groups adjacent to the hydroxyl group limit its antioxidant activity.
BHA is a mixture of two isomers: 3-tertiary-butyl-4-hydroxyanisole and 2-tertiary-butyl-4-hydroxyanisole. The 3-isomer predominates, making up approximately 90% of the total. BHA, like BHT, is a hindered phenol but less so. However, the hindrance of the phenol does provide some favorable properties to both BHA and BHT. Because the phenol is somewhat protected, BHA and BHT exhibit “carry-through” properties in baked and fried foods. This means that they do not fully degrade during heating processes, and therefore continue to protect the food from oxidizing after it has been processed. BHA is sold as white waxy tablets and is often used in combination with BHT.
TBHQ has two hydroxyl groups that give it greater antioxidant activity than BHA or BHT. TBHQ has good carry-through properties in baked and fried foods, and does not cause discoloration upon reaction with metals such as iron. It is often added to polyunsaturated oils used for deep fat frying.
Propyl gallate is the rc-propyl ester of gallic acid. Because of the presence of the three hydroxyls, gallic acid esters make very effective antioxidants. Free gallic acid would presumably make an effective antioxidant but its solubility in lipids is low. Thus, it is esterified with propionic acid to increase lipid solubility. Propyl gallate is degraded by heat and therefore provides poor carry-through properties in foods that are baked or fried. BHA or BHT is often used in combination with propyl gallate to provide carry through properties to baked goods.
2. Chelating Agents. Chelating agents are frequently added to food to retard lipid oxidation. The most common chelating agents added to foods are ethylenediaminetetraacetic acid (EDTA) and citric acid. Many of the phenolic compounds in foods also function as chelating agents in addition to their radical scavenging actions.
Date added: 2023-01-09; views: 552;
