Food Antioxidants. Oxidation in biological systems. Oxygen’s Role in Energy Metabolism
Cellis in our bodies are constantly bombarded by highly reactive chemical species known as free radicals. Free radicals are products of normal metabolism but they also form when radiation strikes our bodies. They are present in high concentrations in cigarette smoke. Free radicals can cause damage to cellular components that may eventually lead to heart disease, cancer, arthritis, and cataracts. Biochemists have shown convincingly that substances called antioxidants can effectively eliminate (or scavenge) reactive free radicals in cells.
This led to the hypothesis that diets high in antioxidants may protect us from developing these serious chronic diseases. Over the last few years, numerous epidemiological, biochemical, and nutritional studies have lent support to this hypothesis and prompted nutritionists and other health professionals to promote diets that are rich in antioxidants. One of the most widely publicized of these studies was authored by Gladys Block and her colleagues at the University of California-Berkeley.
They reviewed about 200 epidemiological studies on the role of dietary fruits and vegetables in cancer risk. In almost all of the studies, high intakes of fruits and vegetables were associated with reduced risk for cancer. This fits with the foregoing hypothesis because fruits and vegetables are particularly good sources of antioxidants. It is important to keep in mind, however, that other compounds in fruits and vegetables could influence cancer without inhibiting oxidative reactions.
Oxidation in biological systems. To understand the significance of antioxidants in foods and how they work, we must first understand what oxidation is and why some forms of oxidation can be damaging to key cell components. This section provides a brief overview of the complex topic of oxidation as it occurs in foods and living biological systems. Please see the glossary for definitions of oxidation, reduction, reactive oxygen species, and free radicals.
A. Oxygen’s Role in Energy Metabolism. Early life on planet earth evolved in an oxygen-free environment until photosynthetic organisms appeared on the scene and began generating molecular oxygen (O2). Once oxygen was present in the environment, organisms evolved mechanisms for using oxygen to more efficiently generate ATP from fuel molecules (e.g., carbohydrates and lipids). ATP is a high-energy molecule used by cells and organisms as the primary energy source for driving muscle contraction, the synthesis of biomolecules (e.g., proteins, DNA, phospholipids, hormones), and the active transport of molecules and ions in and out of cells.
Most of the ATP produced in the oxidation of fuel molecules is generated when electrons flow from NADH and FADH2 to O2 via a series of electron carriers that make up the electron transport chain in mitochondria. The process of generating ATP from electron transfer is called oxidative phosphorylation. NADH and FADH2 are generated in glycolysis, fatty acid oxidation, and the citric acid cycle. When NADH is oxidized to NAD+, two electrons are released for transfer to O2. Clearly, oxidation in this case involves both a loss of electrons and a loss of hydrogen.
The electron transport system in cells is designed to transfer two electrons to each atom of oxygen so that the resulting reduced oxygen can combine with protons to yield harmless water:
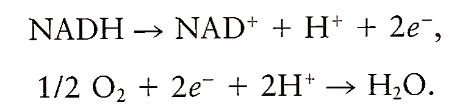
Date added: 2023-01-09; views: 840;
