Basis of Free Radical Injury. Biological Sources of Free Radicals. Ischemia-Reperfusion
A. Biological Sources of Free Radicals. I. Respiratory Burst. Upon exposure to a stimulus, phagocytes start consuming large amounts of oxygen, an event termed “oxidative burst” that results in the generation of reactive oxygen species. Whereas resting phagocytes produce minimal levels of superoxide, those exposed to a stimulus may produce 100 million superoxide molecules per second per cell. Superoxide and H202 are two main oxygen species produced during this process.
These two react in the presence of metal ions to generate more potent radicals like hydroxyl radical, which is so reactive that it reacts with everything that is close to it. Phagocytes are capable of producing large levels of H202 to oxidize intracellular components in erythrocyte targets, to destroy tumor cells, to injure endothelial cells, and to damage leukocyte functions. The H202 also participates in myeloperoxidase-catalyzed oxidation of halides, resulting in the formation of hypohalous acid, most notably hypochlorous acid (HOCl).
The products thus formed have antimicrobial activity. The multicomponent NADPH oxidase located in the plasma membrane of neutrophil catalyzes the formation of superoxide. The superoxide then dismutates to H202. In an inherited condition called chronic granulomatous disease, granulocytes fail to produce superoxide, which is the result of diminished levels of NADPH oxidase.
In addition to their antimicrobial activity, the reactive oxygen species generated during an oxidative burst are also found to be responsible for host tissue injury and inflammation. The symptoms of tissue damage, are redness, swelling, pain, and loss of function. The synovial fluid in the swollen knee joints of rheumatoid arthritis patients contains large number of activated neutrophils. The reactive oxygen species produced by these neutrophils may contribute to the joint injury. The HOCl produced during an oxidative burst by phagocytes can react rapidly with amino acids, sulfhydryl groups, thioesters, amines, and other unsaturated carbon centers. HOCl also inactivates ai-antiproteinase, the major circulating inhibitor of serine proteinases in body fluids, resulting in the uncontrolled action of several proteinases.
Nitric oxide is another free radical produced by several types of cells including macrophages. This radical molecule plays diverse roles as a vasodilator, neurotransmitter, and immunomodulator. NO' is synthesized as a result of five-electron oxidation of arginine catalyzed by nitric oxide synthase (NOS). The macrophages have a form of NOS that is inducible by a stimulus, and upon activation, for example, exposure to microbes, they are shown to produce high levels of NO’. Murine macrophages produce nitrite (N02-) and nitrate (N03-), the stable end products of NO' oxidation, in response to in vitro treatment with lipopolysaccharide and cytokines. NO' synthesis is increased during inflammation and the symptoms of inflammation can be treated with NOS inhibitors.
2. Ischemia-Reperfusion. Ischemia-related pathological processes account for the majority of deaths in the United States and a burst of oxygen-derived free radical formation is at least partially responsible for the damage that results. Although the mechanisms for cellular and tissue injury during ischemia-reperfusion are complex and variable, a representative scenario begins with cellular energy depletion as a result of ATP breakdown. This energy depletion results in calcium influx, which in turn activates endogenous proteases leading to the conversion of xanthine dehydrogenase to xanthine oxidase. These enzymes are two forms of the same gene product. Whereas xanthine dehydrogenase utilizes NAD+ as an electron acceptor, xanthine oxidase uses O2 as an electron acceptor. Upon reperfusion, O2 reenters the tissue and gets reduced to large amounts of 02- by xanthine oxidase.
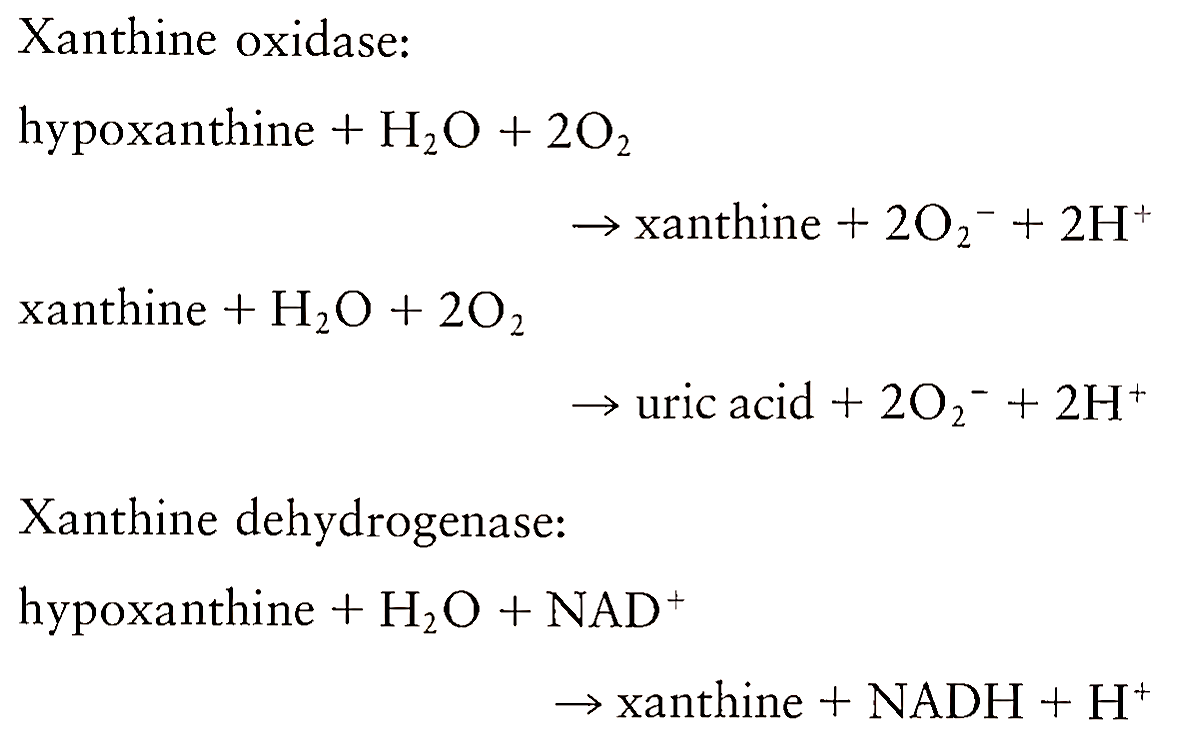
Xanthine oxidase was the first documented biological source of superoxide. Superoxide thus produced, in collaboration with H202 and transition metal ions, initiates the formation of more potent secondary radicals that cause the tissue damage. Thus ischemia itself does not bring about this tissue injury, rather the injury takes place during the reoxygenation process.
Evidence has also been accumulated to show that neutrophils are another source of tissue injury during ischemia-reperfusion. Administration of free radical scavengers such as superoxide dismutase (SOD), catalase, and dimethylthiourea has been found to inhibit the infiltration of neutrophils into reoxygenated tissues, suggesting that superoxide radical directly or indirectly initiates the infiltration of neutrophils into tissues during the postischemic period. These neutrophils, which contain the free radical-producing enzymes NADPH oxidase and myeloperoxidase, trigger the tissue damage already initiated by xanthine oxidase.
Depletion of neutrophils has been shown to significantly attenuate postischemic parenchymal and microvasculature dysfunction. The injury resulting from free radical generation will be more pronounced because of the observed drop in antioxidant defenses during ischemia-reperfusion.
3. Mitochondria. The mitochondrion is another source of production of free radicals. The major cause of radical production in mitochondria is the partial reduction of O2 by electrons leaking from the electron transport chain. Superoxide is the main oxygen species generated by mitochondria. The rate of superoxide radical production is highest when the components of the electron trans port chain are in the reduced state. More than 90% of O2 consumed by the human body is used by mitochondrial cytochrome oxidase, of which only 2-5% goes toward the production of reactive oxygen species.
The reactive oxygen species production takes place mainly at complexes I, II, and III of the respiratory chain as a result of one-electron reduction of O2. Superoxide thus generated undergoes dismutation to H202 by manganese superoxide dismutase (MnSOD). It has been demonstrated that the rate of oxygen species generation rises with an increase in 02 concentration. Factors that enhance the leakage of electrons from the respiratory chain include several pathological events such as ischemia-reperfusion and sepsis.
Date added: 2023-01-09; views: 646;
