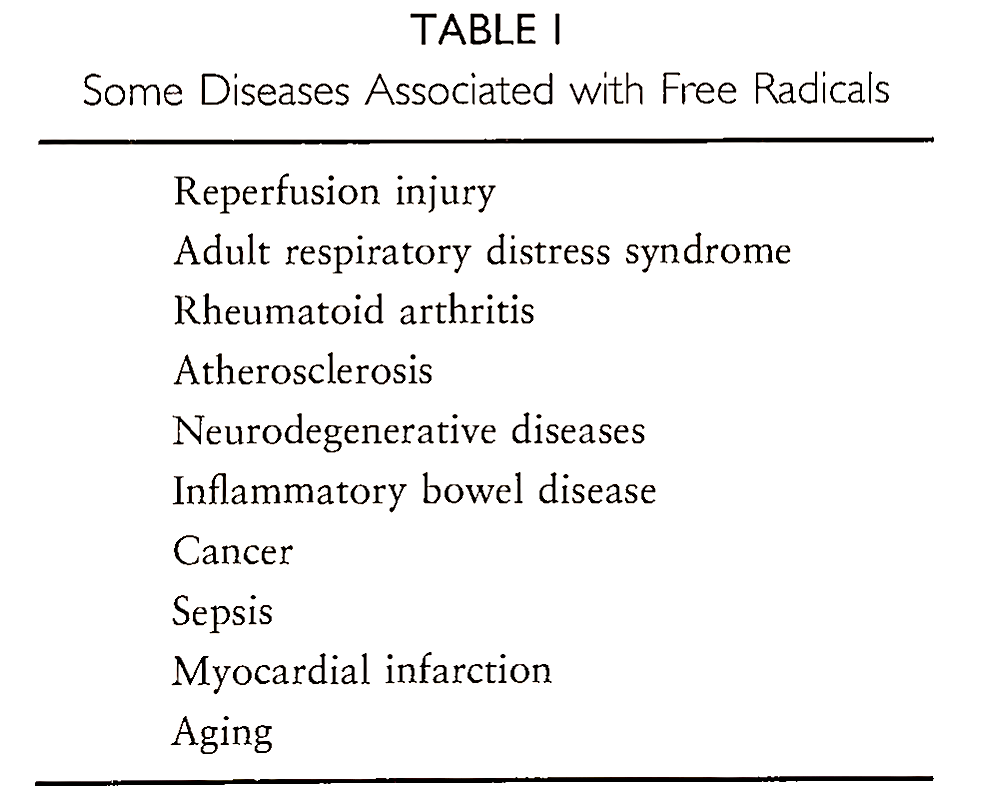
Diseases Associated with Free Radical Injury
Although increased accumulation of radical species has been observed in a number of diseases (Table I), the exact role played by these species is not yet clearly understood.
A. Rheumatoid Arthritis. In rheumatoid arthritis, thickening of synovial lining takes place, resulting in folds and infiltration by blood vessels and inflammatory cells. The synovial fluid from the knee joints of rheumatoid arthritis patients has been found to contain higher levels of lipid peroxidation products. The hyaluronic acid synthesized by rheumatoid synovium is responsible for the viscosity of synovial fluid and is susceptible to breakdown by interaction with free radicals.
It has been demonstrated that synovial fluid from rheumatoid arthritis patients contains degraded hyaluronic acid, implicating that oxygen species are responsible for this disease. How are oxygen species generated in rheumatoid arthritis? Radical species may be produced by activated macrophages in the inflamed synovial membrane and by activated neutrophils in the synovial cavity.
B. Adult Respiratory Distress Syndrome. Adult respiratory distress syndrome (ARDS) is a form of acute lung injury, causes of which are varied, such as hypoxia, sepsis, and polytrauma. The mortality rate of ARDS is more than 50%. This syndrome has been shown to be mediated by inflammation as a result of the presence of neutrophils, blood platelets, and other inflammatory mediators in the biopsy specimens of ARDS patients. Increased levels of myeloperoxidase and oxidized antiproteinase in bronchoalveolar lavage fluids and exhalation of excess H202 in the breath of ARDS patients are indicative of involvement of radical species.
No treatment is currently available that is directly aimed at the pathogenic factors of this disease. All attempts at present are directed toward developing strategies to prevent early activator processes, such as injecting antibodies directed against bacterial lipopolysaccharide and tumor necrosis factor.
C. Sepsis. Septic shock is manifested by a dramatic drop in blood pressure as a result of severe infection with gramnegative bacteria. This is a deadly disease that results in failure of various organs such as liver, kidney, and heart, ultimately leading to death. According to one estimation, about 200,000 patients are prone to septic shock annually in the United States alone. It has been demonstrated in animals that endotoxin and cytokines such as tumor necrosis factor and interferon reproduce many of the cardiovascular features of sepsis.
The severe hypotension is caused by the excessive production of NO- induced by the bacterial endotoxin. The bacterial endotoxin or lipopolysaccharide triggers the release of various cytokines, which in turn up-regulate the inducible form of NO- synthase. These cytokines are also responsible for liver failure and cirrhosis. The endotoxin-mediated hypotension is associated with increased NO- synthesis by vascular endothelial cells. Elevated levels of circulating N02- and N03-, the stable products of NO- oxidation, have been documented in septic shock patients.
L-NMMA (N-monomethyl-L-arginine) an inhibitor of NO- synthase, is shown to prevent the endotoxin shock in animal models and has been used successfully to restore normal arterial tension in septic shock patients. Further investigations are directed at the usefulness of NO- synthase inhibitors in treating sepsis because of the serious side effects associated with them.
Bibliography. Grisham, M. B. (1994). “Reactive Metabolites of Oxygen and Nitrogen in Biology and Medicine.’ R. G. Landes Company, Austin, Texas.
Halliwell, B., Gutteridge, J. M. C., and Cross, C. E. (1992). Free radicals, antioxidants, and human disease: Where are we now? J. Lab. (dlin. Med. 119, 598.
Ignarro, L., and Murad, F. (eds.) (1995). “Nitric Oxide: Biochemistry, Molecular Biology, and Therapeutic Implications,” Advances in Pharmacology 34. Academic Press, San Diego.
Janssen, Y. M. W., Houten, B. V., Borm, P. J. A., and Mossman, B. T. (1993). Biology of disease: Cell and tissue responses to oxidative damage. Lab. Invest. 69, 261.
Kehrer, J. P. (1993). Free radicals as mediators of tissue injury and disease. Crit. Rev. Toxicol. 23, 21.
Kerrigan, S. L., and Stotland, M. A. (1993). Ischemia reperfusion injury: A review. Microsurgery 14, 165. Kerwin,J.F., Lancaster, J.R., Jr., and Feldman, P. L. (1995).,Nitric oxide: A new paradigm for second messengers. J. Med. Chem. 38, 4343.
Lancaster, J. R., Jr. (1992). Nitric oxide in cells. Am. Sci. 80, 248.
McCord, J. M. (1993). Human disease, free radicals, and the oxidant/antioxidant balance. Clin. Biochem. 26, 351.
Packer, L. (ed.) (1996). Nitric oxide: Physiological and pathological processes. Meth. Enzymol. (Part B) 269.
Pryor, W. A., and Squadrito, G. L. (1995). The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 268, L699-L722.
Date added: 2023-01-09; views: 689;
