Plant Foods. Structures of phytoalexins
Plant foods are essentially a combination of chemicals. Some of these chemicals are required nutrients, or nonnutrients that may inhibit diseases such as coronary thrombosis or cancer, but some may be toxicants. Many toxicants are present in either a free or a bound state (e.g., glycosides). There are still many natural chemicals in every plant that have not been completely identified. Indeed, there are probably one or more toxicants naturally occurring, albeit at low levels, in every plant food depending on various environmental, genetic, and as yet unknown factors.
A common vegetable may have a level of toxicant so small that it is normally metabolized and excreted by the consumer without any effect, or the food is never consumed in continuous or high enough levels to observe either acute or chronic toxic symptoms. In addition, a known toxicant that is in specific strains of a common vegetable can be bred out by either traditional plant breeding or by genetic engineering.
For example, a black Puerto Rican variety of lima bean yields about 300 mg of cynanide per 100 g of seed. This is fatal for an adult human who consumes 100 g of these lima beans. Serious incidences of cyanide poisoning occurred in Europe in the early part of the twentieth century from these types of tropical imported lima beans. Present-day strains of lima bean contain under 10 mg of HCN per 100 g of seed, which permits a rather large margin of safety for all consumers.
Important, common food plants may be related to very toxic poisonous plants. For example, the common tomato and potato are both members of the plant family Solanaceae. This family includes belladonna (deadly nightshade), which produces atropine; jimson weed, which produces scopolamine; henbane and mandrake, which produce atropine and other tropane alkaloids; tobacco, which produces nicotine; ground cherry, which produces solanine (a toxic glycoalkaloid, Fig. 3c); and trumpet flowers and hairy nightshade, which produce solanaceous alkaloids. All of these chemicals are extremely toxic, but some (i.e., atropine and scopolamine) are useful medicinals.
The vine and unripe fruit of the tomato and potato leaves, green tubers, or tuber sprouts can have acutely toxic levels of solanine (see Fig. 3c), similar to the level (in excess of 1000 mg/kg of fresh plant material) occurring in nonfood, poisonous plant members of Solanaceae. It should be pointed out that a number of reported potato glycoalkaloid poisonings of people, especially in Europe, occurred in the early part of the twentieth century. The United States has not documented a single poisoning in the past 50 years, even though the consumption of potatoes and potato products averages about 125 pounds per year. Continued analyses of potatoes, especially new varieties, in the United States and Europe indicate that tubers have much less than 200 mg/kg fresh tuber, the maximum level of safety.
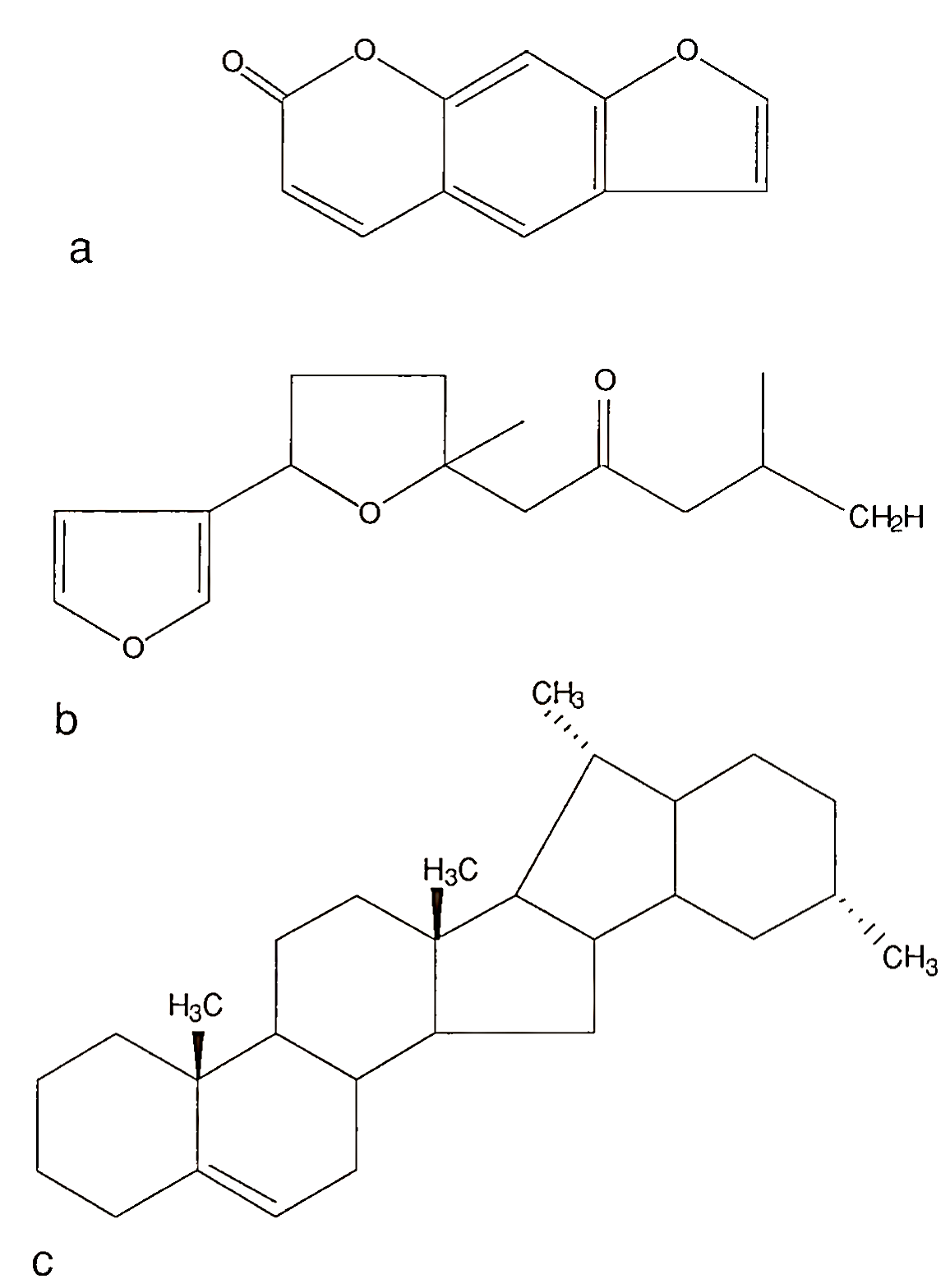
FIGURE 3. Structures of phytoalexins: (a) psoralen; (b) ipomeamarone; (c) solanine
Phytoalexins, called stress metabolites, are synthesized toxic compounds that are thought to be needed for the plant’s defense against pathogens and physical damages. These compounds exhibit toxicity not just to pathogenic microorganisms but also to insects, animals and humans, dependent, of course, on the amount present. Various stimuli, such as bacterial or viral infection, cold, ultraviolet light, heavy metals, fungicides, herbicides, or nematode attack, can contribute to pytoalexin production.
Thus, a number of these toxic phytoalexins can be described as “natural pesticides.” The toxicologic aspects of most phytoalexins have received little attention. Examples of three phytoalexins (Fig. 3) are: furanocoumarins, such as psoralen (celery and parsley), which causes phototoxic effects on the skin of harvesters and handlers; ipomeamarone (sweet potato), which causes liver degeneration and pulmonary edema in animals; and solanine (potato), which is a human plasma cholinesterase inhibitor and animal teratogen. Presently, there are more than 20 food plants known that can synthesize one or more phytoalexins during times of stress.
One or more plant genes can express naturally occurring toxicants, an example of which was observed in Australia during the early 1980s. Many individuals, within a few weeks, reported stomach cramps, diarrhea, vomiting, and headaches after consuming zucchini squash, which was also described as unusually bitter. Purgative bitter principals have been previously known, for at least 30 years, in various nonfood plants within the family Cucurbitaceae, the same family of zucchini squash. Tetracycline triterpenes, known as cucurbitacins (Fig. 4, cucurbitacin E) and studied some years earlier as a possible drug with purgative, emetic, and narcotic properties but abandoned owing to their high acute toxicities, were present in the toxic squash.
Bitter zucchini squash was later found in a few plants growing in Alabama gardens and California fields. Production of this squash was concluded to be the result of accidental, aberrant outcrossings in seed-producing fields. Great care is now taken in marketing commercial squash seeds.
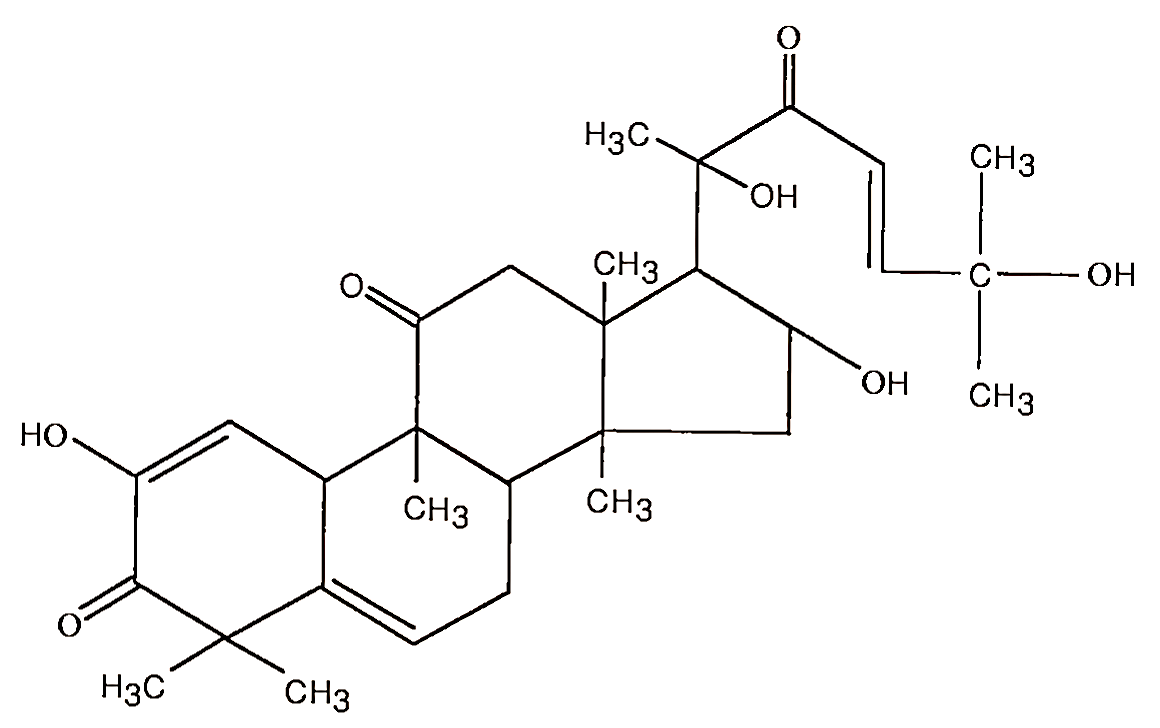
FIGURE 4. Structure of cucurbitacin E
Plant breeders, either by traditional breeding methods or by modern recombinant DNA methodologies, purposely develop disease- and insect-resistant plant foods. These crops then require reduced, or even no, chemical pesticide applications. However, in many cases, there is a lack of knowledge of potential toxicants present in the newly developed hybrid food plant. One recent example is a virus-resistant hybrid lettuce developed from crosses with wild, nonfood lettuce strains, Lactuca saligna and L. virosa.
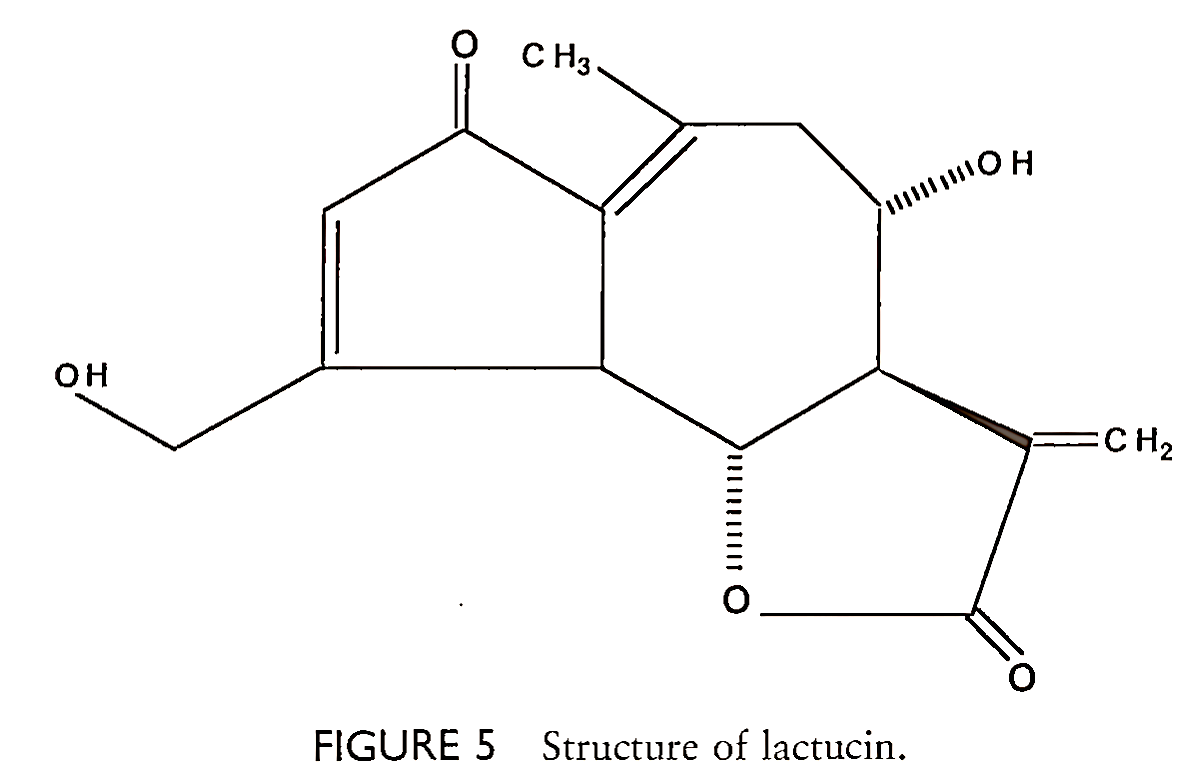
This development was extremely important since there are no chemical pesticides efficacious against plant viruses. The newly developed hybrid lettuce was grown, marketed, and consumed without any apparent ill effects. However, the wild-type lettuce strains that were used in the traditional breeding project to produce the hybrid lettuce have been known for many years to contain high levels of sesquiterpene lactones, known as lactucins (Fig. 5), which have antibiotic, cytotoxic, and allergenic properties. Fortunately, later analyses of the hybrid lettuce strains showed quite small amounts of sesquiterpene lactones. Although lactucins may or may not be the effective natural compound that causes viral resistance, the levels of these compounds, with known detrimental biological effects, should have been of major concern and, at least, quantitated prior to commercial, public release.
An “Integrated Breeding and Environmental Strategy” program was suggested some years ago for breeders and developers of vegetables, cereals, fruit, and oil-bearing crop plants that could or may possess possible natural toxic compounds. An evaluation of these recognized toxic substances, including safety assessment, would be accomplished prior to market release. For the most part, with some exceptions, this has never been done, probably because of the costs and lack of specific enforced regulations.
Some natural food toxicants can show numerous toxicologic effects and yet may have potential health values. Glucosinolates (Fig. 6) are natural plant bioactive organosulfur compounds in Cruciferae vegetables (e.g., cabbage, broccoli, brussels sprouts). There are about 100 known glucosinolates in the plant kingdom, 10-12 distinct vegetable types, that are enzymatically metabolized to various compounds. These metabolites, studied over the past 50 years, have shown diverse biological effects from goiter development to anticarcinogenic properties in experimental animals. One metabolite, indole-3-carbinol from the breakdown of 3-indolylmethyl glucosinolate (Fig. 6b), seems promising as a potential preventive agent against human breast and uterine cancers.
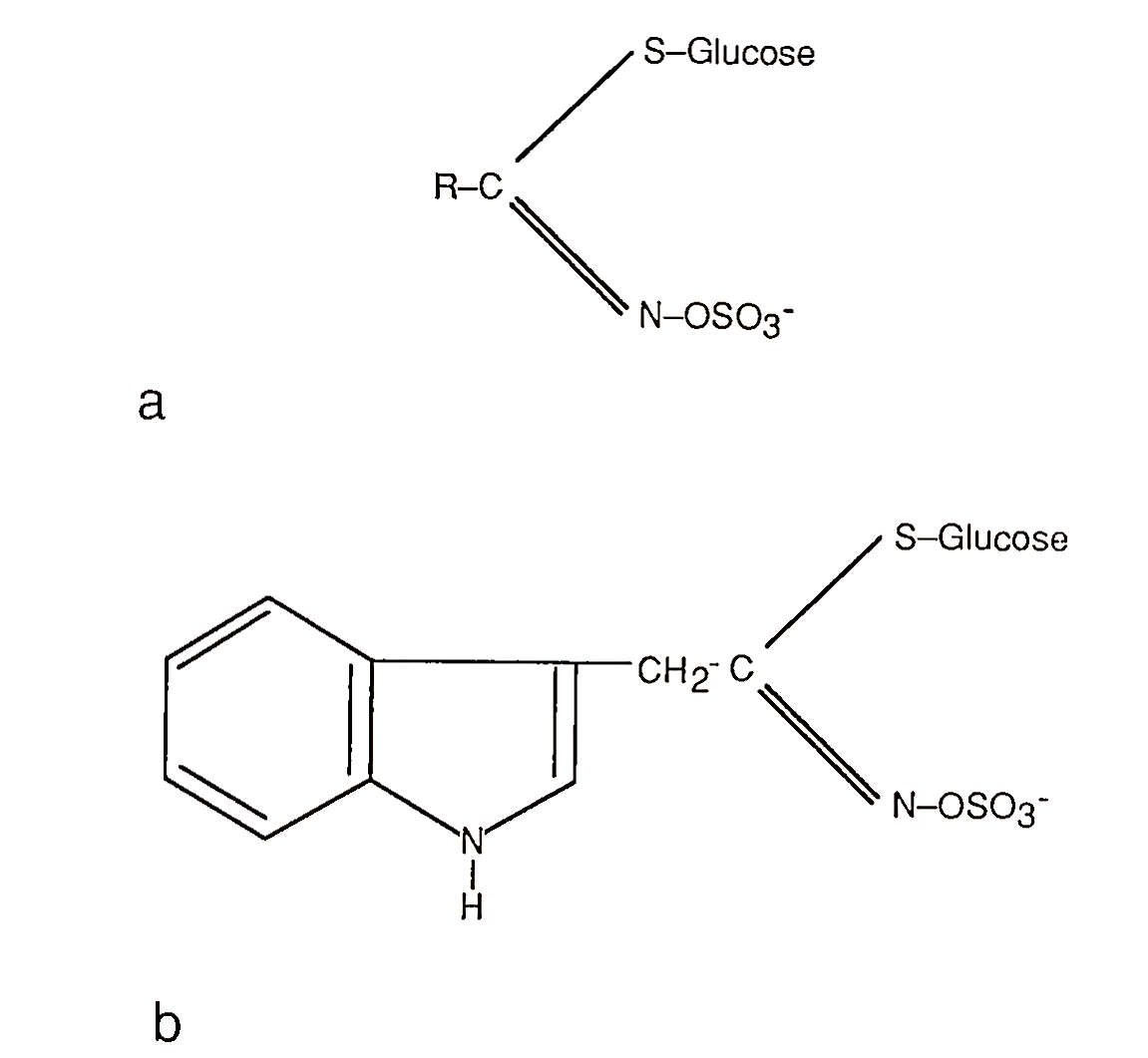
FIGURE 6. Glucosinolates: (a) general structure and (b) structure of 3-indolymethyl glucosinolate
Bibliography. Bier, R. C., and Nigg, H. N. (1994). Toxicology of naturally occurring chemicals in food. In “Foodborne Disease Handbook,” (Y. H. Hui, J. R. Gorham, K. D. Murrell, and D. O. Cliver, eds.), Vol. 3. Marcel Dekker, New York.
Halstead, B. W. (1994). Fish toxins. In “Foodborne Disease Handbook” (Y. H. Hui, J. R. Gorham, K. D. Murrell, and D. O. Cliver, eds.), Vol. 3. Marcel Dekker, New York.
Hathcock, J. N. (1996). Safety limits for nutrients. J. Nutr. 126, 2386S.
Shibamoto, T., and Bjeldanes, L. F. (1993). “Introduction to Food Toxicology.” Academic Press, San Diego.
Stoewsand, G. S. (1995). Bioactive organosulfur phytochemicals in Brassica oleracea vegetables—A review. Food Cbem. Toxicol. 33, 537.
Tamaki, H., Robinson, R. W., Anderson, J. L., and Stoewsand, G. S. (1995). Sesquiterpene lactones in virus-resistant lettuce.]. Agric. Food Chem. 43, 6.
Ybanez, N., and Montoro, R. (1996). Trace element food toxicology: An old and ever-growing discipline. Crit. Rev. Food Sci. Nutr. 36, 299.
Date added: 2023-01-09; views: 670;
