Reactions of Free Radicals and Their Targets
The free radicals vary widely in their reactivity toward the tissue target molecules. Superoxide, although a radical, can cause little damage by itself. Rather, it is the reaction of superoxide with other radical species that leads to the formation of more potent oxidizing agents. The dismutation of superoxide either spontaneously or enzymatically catalyzed by SOD generates H202:
![]()
H202 is also produced by several other enzymes such as L-amino acid oxidase, glucose oxidase, and monoamine oxidase. H202 can readily cross the cell membrane and thus take part in reactions away from its site of production. Like superoxide, H202 also has limited reactivity. Simultaneous generation of superoxide and H202 inside the cell has been shown to cause DNA damage, but neither of these two reacts directly with DNA. Both 02 and H202 produced during phagocytosis or ischemia-reperfusion participate in a reaction catalyzed by transition metals to generate hydroxyl radical.
Copper also catalyzes the generation of OH'. Both ferrous and ferric salts react with H202, the ferrous compounds reacting faster. The importance of iron in this type of oxidative damage raises the question of the source of intracellular free iron. The source of iron is not clearly elucidated in most of the circumstances. Almost all of the iron pool within the cell is not in free form as it is sequestered mainly by transferrin, a transport protein, and ferritin, a storage protein, thus acting as antioxidants.
The storage capacity of ferritin is quite high, namely, 4500 mol of iron per mole of protein. Thus, in the absence of free iron, 02 and H202 at physiological concentrations may have limited damaging effects. The blood plasma of healthy humans is found to contain no free iron and copper. Targe amounts of 02 formed during an oxidative stress mobilize iron from ferritin. Intracellular degradation of ferritin also leads to the liberation of iron. Hydrogen peroxide can degrade heme proteins to release iron and thereby promote cell injury.
The major biological targets of free radicals are deoxyribonucleic acids, unsaturated lipids, and proteins. Neither H202 nor 02- can cause a DNA strand break, but hydroxyl radicals can cause cellular damage by nucleic acid base modification and DNA strand scission. The DNA strand scission takes place as a result of hydroxyl radical interaction with the sugar-phosphate backbone. Reaction with thymidine also generates lesions, producing single-stranded breaks.
Thus the appearance of single-stranded DNA breaks is an indication of the interaction with free radicals. This strand scission can be prevented using hydroxyl radical scavengers. Another mechanism of DNA damage during an oxidative stress is due to rises in intracellular free Ca2+, which in turn activates nucleases, leading to the fragmentation of DNA.
Membrane lipids are another class of biomolecules that are susceptible to free radical attack. The peroxidation of lipids by radical species has been implicated in a variety of tissue injury and disease states. Cell membranes are rich sources of polyunsaturated fatty acids, and lipid peroxidation is defined as the oxidative destruction of polyunsaturated fatty acids present in cell membranes, leading to the formation of lipid hydroperoxides. When a free radical reacts with a nonradical, another free radical is produced, thus initiating a chain reaction. This forms the basis of oxidation of lipid molecules, for example, polyunsaturated fatty acids. The following scheme defines the process of lipid peroxidation:
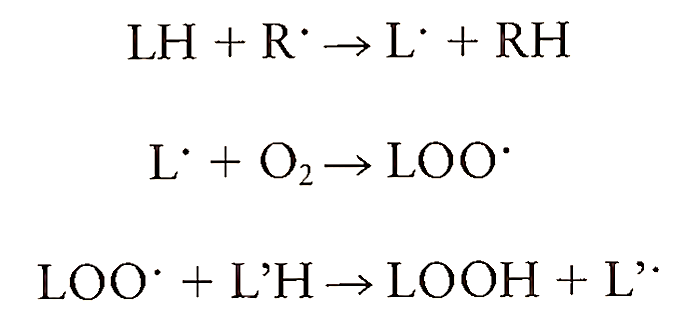
where LH is the lipid molecule, R’ is the radical species, and LOOH is the lipid hydroperoxide. The radicals react with a lipid molecule by abstracting a hydrogen atom and forming a radical. This radical, upon addition of oxygen, generates lipid peroxyl radical, which abstracts a hydrogen atom from another lipid to form a lipid hydroperoxide and another lipid radical. Transition metals like iron and copper participate in lipid peroxidation in two ways. First, they catalyze the formation of initiating species and then they promote peroxidation by reacting with lipid hydroperoxides and decomposing them to peroxyl radicals:
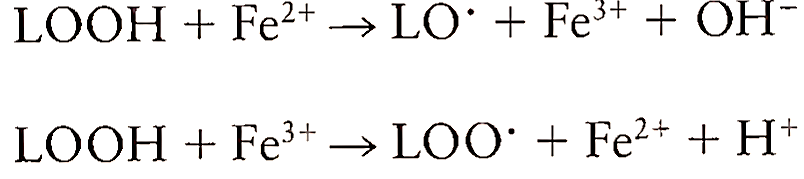
The cascade of free radical formation amplifies the damage done to membrane proteins and DNA. The formation of lipid peroxides within the membrane disrupts its functioning, altering the fluidity and letting the ions such as Ca2+ leak through the membrane. Owing to their hydrophobic nature, lipid radicals almost always react with membrane-associated components. Peroxyl radicals may react with membrane proteins and thus inactivating enzymes and receptors critical to cell function. Examples of this are losses of cytochromes P-450 and glucose-6-phosphatase activity in microsomes. The cell membrane proteins can also be damaged by aldehydes like malondialdehyde and 4-hydroxy-2,3-trarcs-nonenal, the byproducts of lipid peroxidation. These products diffuse away from their site of formation and cause tissue injury at a distant site.
Protein denaturation or damage due to free radical attack is dependent on their amino acid content and the importance and location of susceptible amino acids. Proteins consisting of sulfhydryl groups and unsaturated amino acids are more susceptible to free radical damage and hence proteins containing cysteine, methionine, histidine, and phenylalanine are subject to amino acid modification. This modification in amino acids may then lead to disruption in secondary, tertiary, and quaternary structures of proteins. The requirement of a particular amino acid for the activity of an enzyme or location of an amino acid for the structural integrity determines the susceptibility of proteins to oxidative damage. Hydrogen peroxide and other peroxides oxidize glutathione through the catalytic action of glutathione peroxidase, thus depleting an important antioxidant that is essential for maintenance of the intracellular reducing pool:
![]()
The brain enzyme glutamate synthetase, whose function is glutamate removal, is susceptible to radical damage. Decreased glutamate synthetase activity and increased appearance of protein degraded end products have been observed in brains of older humans.
Nitric oxide and its reaction product with superoxide, peroxynitrite (ONOO-), are responsible for causing inactivation of a number of physiologically important proteins:
![]()
The most widely studied reaction of NO' is its interaction with the iron of heme- and nonheme- containing proteins to form dinitrosyl iron complexes, thereby inactivating the proteins. Thus NO' inactivates aconitase, an enzyme of the tricarboxylic acid cycle, and cytochromes P-450, a class of proteins involved in drug metabolism. NO' also inactivates another hemecontaining enzyme, catalase, which provides protection against oxidative damage by breakdown of H202. Inactivation of critical iron-sulfur (Fe-S)-containing enzymes of mitochondrial respiration takes place due to their reactivity with NO'. In contrast, the interaction of NO' with the heme of guanylate cyclase leads to the stimulation of its enzyme activity.
This results in elevation in cGMP levels and vasorelaxation. One of the widely studied reactions of NO' is the formation of N03- and methemoglobin as a result of interaction between NO' and oxyhemoglobin:
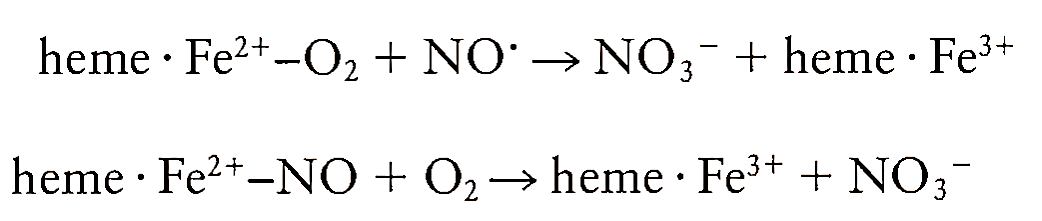
The formation of this nitrosylhemoglobin has been demonstrated during allogenic organ transplantation and vasodilator administration in humans.
The peroxynitrite is not a free radical but an anion formed as a result of interaction between two free radicals. The formation of peroxynitrite thus dramatically increases the toxicity associated with the presence of either 02- or NO alone. The protonated form of ONOO", peroxynitrous acid (ONOOH), decom poses to form N03-. It has been proposed that an activated isomer of peroxynitrous acid, HOONO*, can be formed in the steady state during the decomposition of HOONO and this species has “hydroxyl radical-like reactivity.” The toxic reactivity of peroxynitrite is varied as a potent oxidant as well as a nitrating and hydroxylating agent. It can directly oxidize low-molecular-weight-SH groups such as glutathione and -SH groups in proteins and thus deplete intra- and extracellular antioxidant levels. Reaction of ONOO- with metal ions has been shown to produce a powerful nitrating agent that resembles the nitronium ion (N02+). Thus, the tendency of ONOO- to react with sulfhydryls and metals significantly contributes to the in vivo toxicity of ONOO- by inactivating vital enzymes in the energetic cellular metabolism. Peroxynitrite reacts with phenolic groups such as tyrosine residues in proteins to form nitrotyrosine. The determination of nitrotyrosine has been used as a means of detecting the formation of ONOO-.
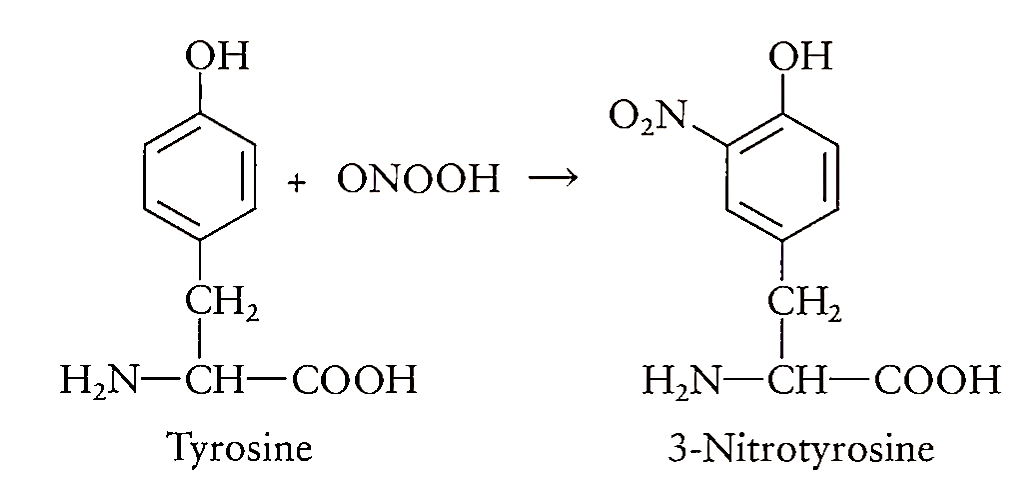
Recently, the reaction of peroxynitrite with carbon dioxide has been brought to attention. This results in the formation of nitroperoxycarbonate anion (0 = N—00C02-), which in turn rearranges to nitrocarbonate anion (02N—C02-). The nitrocarbonate anion thus formed has been proposed to act as an oxidant and nitrating species in biological systems, thus generating peroxynitrite. These observations underline the importance of C02 in the cell culture studies of peroxynitrite. Peroxynitrite has been shown to inactivate a number of enzymes, including superoxide dismutase, ocj-antiproteinase, and prostacyclin synthase. Extensive nitration of tyrosine occurs around alveolar inflammatory cells in patients with respiratory distress syndrome, pneumonia, and sepsis.
Date added: 2023-01-09; views: 643;
