Defenses against Free Radical Toxicity
Free radical species are constantly produced in vivo either accidently or by design. Our body has devised various lines of defenses to guard against the deleterious effects of a plethora of radical species. These defenses (antioxidants) include thiols, enzymes, and transition metal chelators. These antioxidants act to lower the effective levels of intracellular oxidant species, which may otherwise cause cellular and tissue injury.
A. Low-Molecular-Weight Antioxidant. Glutathione (GSH) is the major low-molecular-weight thiol that serves the role as antioxidant. GSH is present at millimolar quantities in most mammalian cells. GSH in cytosol serves as a ready source of reducing equivalents and is released from cells during oxidative stress. It acts by reducing H202, the precursor of hydroxyl radical, to water through the catalytic action of glutathione peroxidase. In this process, glutathione gets oxidized to glutathione disulfide (GSSG). The disulfide is reduced to GSH at the expense of NADPH catalyzed by glutathione reductase:
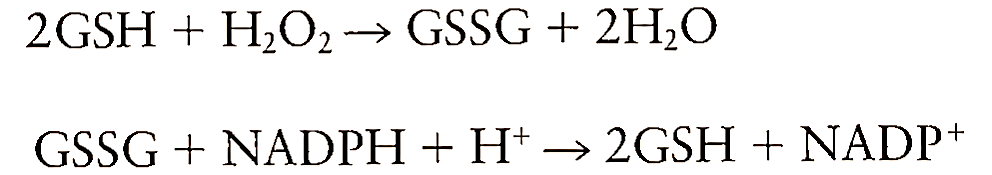
The intracellular ratio of GSH/GSSG must be maintained at significantly higher levels to overcome the damaging effects of peroxides. GSH may also play a role in the protection process by reducing disulfide linkages, by serving as a storage form of cysteine, and by acting as a cofactor for many enzymes. GSH has been suggested to be a protective agent during cardiopulmonary bypass surgery.
Tocopherol (vitamin E) is the primary chainbreaking antioxidant, which acts by trapping peroxyl radical in the hydrophobic milieu. The vitamin E radical formed as a result is fairly stable and hence vitamin E is considered a good antioxidant. Other examples of low-molecular-weight antioxidants include dimethyl sulfoxide, dimethyl thiourea, ascorbic acid, lazaroids, B-carotene, and allopurinol.
B. Enzymatic Antioxidant. Among the enzymatic antioxidants, SODs have been most widely studied and used to treat the injury caused by oxygen radicals. SODs are present in every type of aerobic cell. Their function is to catalyze the dismutation of 02- to H202 and 02:
![]()
These enzymes have a transition metal in the active site, which is copper (Cu2+, Cu+), nonheme iron (Fe3+, Fe2+), or manganese (Mn3+, Mn2+). Eukaryotic cells contain two isozymes of SOD, of which Cu/Zn-SOD is localized mainly in the cytosol and Mn-SOD is synthesized in mitochondria. SOD is widely used to prevent the injury to tissues during ischemia-reperfusion and is also used to treat injury associated with inflammation. It should also be noted that SOD can act as a prooxidant at higher concentrations. Low- molecular-weight SOD mimics have been synthesized to overcome the problem associated with SOD permeability through cell membrane and its high cost of production.
Catalase is another radical-scavenging enzyme that acts in concert with SOD to combat the lethal effects of 02-. The H202 produced by SOD becomes a substrate for catalase and gets degraded to H20:
![]()
Thus, catalase plays an important role in detoxification, since SOD alone might elevate the levels of,H202 and generate hydroxyl radicals through a Haber-Weiss reaction. Many animal studies have been carried out with the combined administration of catalase and SOD to treat ischemia-reperfusion injury. Catalase is present in all kinds of mammalian cells and is localized in the peroxisomes and microperoxisomes.
Glutathione peroxidase reduces not only H202 but also other hydroperoxides by using GSH as a reducing agent and forming glutathione disulfide. The disulfide is then reduced back to GSH through glutathione reductase at the expense of NADPH, thus replenishing the levels of GSH lost during the degradation of peroxides:
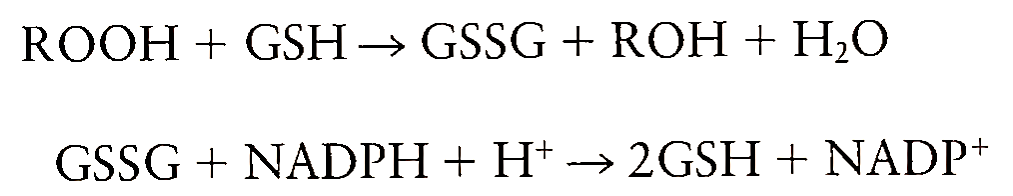
There are two isoforms of glutathione peroxidase, one of which requires selenium as a cofactor. The selenium-dependent isoform reduces both lipid hydroperoxides and H202, whereas the selenium-inde pendent isoform reduces only H202.
C. Transition Metal Chelator. Transition metal chelators do not directly interact with free radicals, instead they provide protection by chelating the metal ions that catalyze the formation of free radicals. Iron is the major transition metal that participates in the free radical formation. Deferoxamine is the most commonly used iron chelator to scavenge the oxygen radicals. Other iron chelators like ethylenediaminetetraacetic acid (EDTA) and nitrilotriacetic acid (NTA) potentiate the toxicity. Deferoxamine is a specific chelator of Fe3+ and can be injected into humans and experimental animals. It is a bacterial siderophore isolated from the species Streptomyces pilosus. Deferoxamine administration has been widely used in treatment of diseases associated with iron overload like thalassemia and to limit the injury during ischemia-reperfusion. The sensitivity of isolated rat hepatocytes to H202 killing has been reduced by pretreating the cells with deferoxamine. In addition to its role as a metal chelator, deferoxamine has been recently shown to scavenge ONOO-. Hence its protective action during oxidative injury does not necessarily imply that it is acting by chelating iron.
Date added: 2023-01-09; views: 618;
