Reactions of Free Radicals in the Body. Lipid Peroxidation
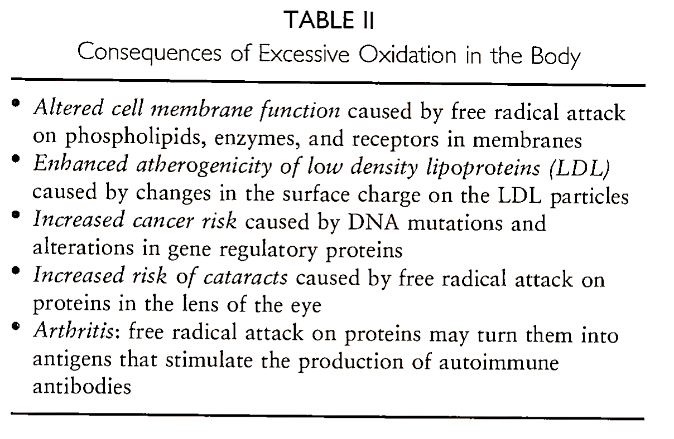
Since free radicals are unstable and seek another electron to combine with their unpaired electron, they may attack other molecules in their vicinity. This can have serious consequences if the molecules that are attacked are altered in such a way that they no longer function normally. We now know that free radicals may attack nucleic acids, proteins, carbohydrates, and lipids, all molecules with vital roles in the cell (Fig. 1). It is widely believed that free radical damage to these molecules can lead to cancer, heart disease, cataracts, and other chronic diseases associated with aging (Table II).
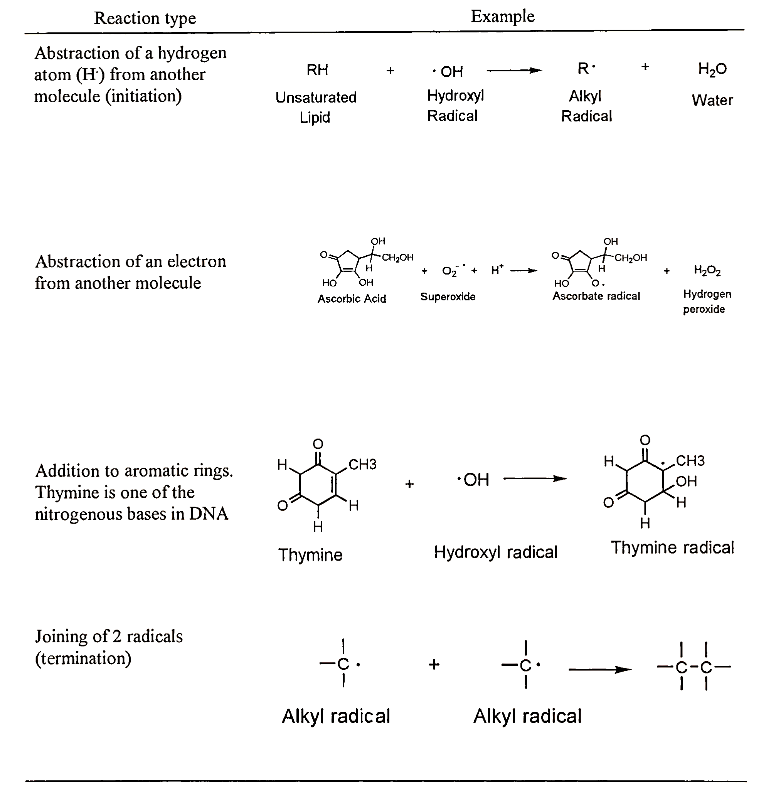
FIGURE I. Free radical reactions that may occur in body cells and/or foods
Lipid Peroxidation. To understand how antioxidants work, we must first understand how free radicals cause oxidation in the first place. As mentioned earlier, free radicals can damage DNA, proteins, carbohydrates, and lipids. We will use the example of lipid oxidation since it is a significant problem both in foods and in the body.
Lipid peroxidation (the oxidation of lipids) has been the subject of intense research for several decades because of its importance in foods and, more recently, its putative role in heart disease and cancer. Products of lipid peroxidation in foods include volatile aldehydes that often have unpleasant odors and flavors. Lipid peroxidation is a major cause of food deterioration and a great deal of effort has gone into developing strategies for reducing or preventing it. The process of lipid peroxidation is summarized in the following and in Fig. 2.
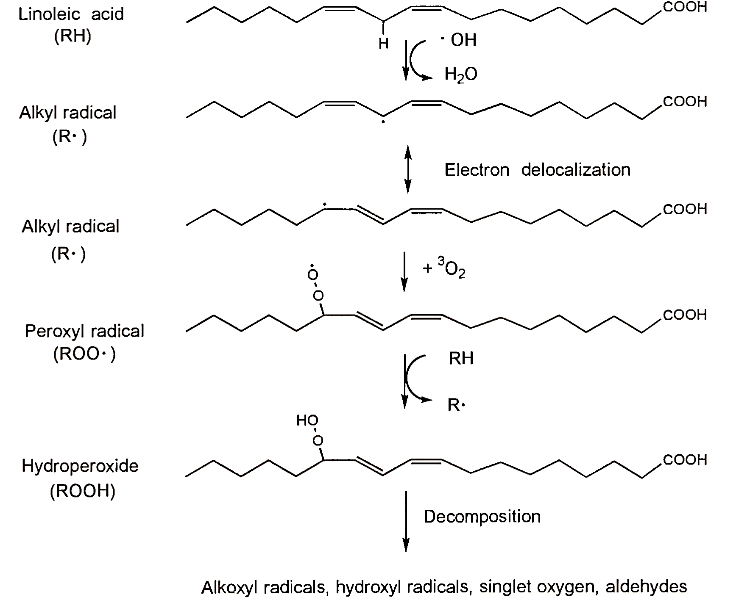
FIGURE 2. Lipid peroxidation of linoleic acid initiated by a hydroxyl radical. RH represents another unsaturated fatty acid
To begin the process of lipid peroxidation, a free radical must be generated. This step is often called the initiation step or simply initiation. As described in the foregoing, free radicals may be generated from the decomposition of lipid hydroperoxides (to form peroxyl or alkoxyl radicals), water (to form hydroxyl radicals), and hydrogen peroxide (to form hydroxyl radicals) or by the generation of superoxide.
Once a free radical is formed, it can abstract a hydrogen atom from chemical compounds containing hydrogens. This occurs fairly readily in unsaturated fatty acids because the hydrogens on carbons adjacent to a carbon-carbon double bond are susceptible toabstraction. Hydrogens on saturated fatty acids are much harder to remove so peroxidation of saturated fatty acids does not occur to an appreciable extent.
When a hydrogen atom (H-) is removed from a fatty acid, an alkyl radical (R-) forms. Alkyl radicals readily react with triplet oxygen to form peroxyl radicals, RO2-, which in turn can abstract a hydrogen from another unsaturated fatty acid to form a hydroperoxide and another alkyl radical. Thus, a chain reaction is set up because the initial reactant, R-, is regenerated. Moreover, additional radicals may be generated by the decomposition of the lipid hydroperoxides. Thus, the potential damage caused by free radicals is much greater than would be expected if chain reactions were not involved. Presumably, lipid peroxidation will continue indefinitely once it gets started as long as oxygen and unsaturated lipids are available and free radicals continue to be generated.
Date added: 2023-01-09; views: 732;
