Reactive Oxygen Species: Free Radicals. Reactive Oxygen Species: Nonradicals
Reactive oxygen species (ROS) are oxygen-containing species that are more reactive than ground-state molecular oxygen. Biologically significant ROS include most oxygen free radicals, hydroperoxides, and singlet oxygen.
Enzymes in the electron transport chain have tight control over the transfer of electrons from NADH and FADH2, ensuring complete reduction of O2 to H2O. However, the process is not 100% efficient and one-electron transfers may also occur, resulting in the formation of a free radical called superoxide anion:
![]()
Another source of free radicals is the homolytic cleavage of an O-H bond in water. This may occur when ionizing radiation strikes the water in our bodies or in food (we are constantly exposed to low levels of radiation from naturally occurring radioisotopes):
![]()
Hydroxyl radicals may also form from the decomposition of hydrogen peroxide in a reaction catalyzed by iron ions:
![]()
Another type of free radical called alkoxyl radicals form when transition metals such as iron catalyze the decomposition of lipid hydroperoxides:
![]()
Lipid hydroperoxides are products of lipid peroxidation (see Section II) Most free radicals are highly reactive species. They may attack a variety of chemical compounds within the cell to obtain another electron to pair up with the unpaired electron, that is, they oxidize compounds by removing an electron or radical.
Molecular oxygen is also a free radical. It contains two unpaired electrons in separate orbitals and exists in the so-called triplet state. The chemical formula for molecular oxygen is sometimes written as 302 to emphasize the fact that it is in the triplet state. Triplet oxygen needs to gain two electrons to achieve nonradical status. Surprisingly, 302 does not easily react with most biological molecules without the aid of specific enzymes. This is because its two unpaired electrons have the same spin state, making it energetically unfavorable to accept a pair of electrons with opposite spins (most electrons in biological molecules exist in pairs with opposite spins). Triplet oxygen does react readily with other free radicals to form new radicals (see the following). Other free radicals include alkoxyl and peroxyl radicals (Table I).
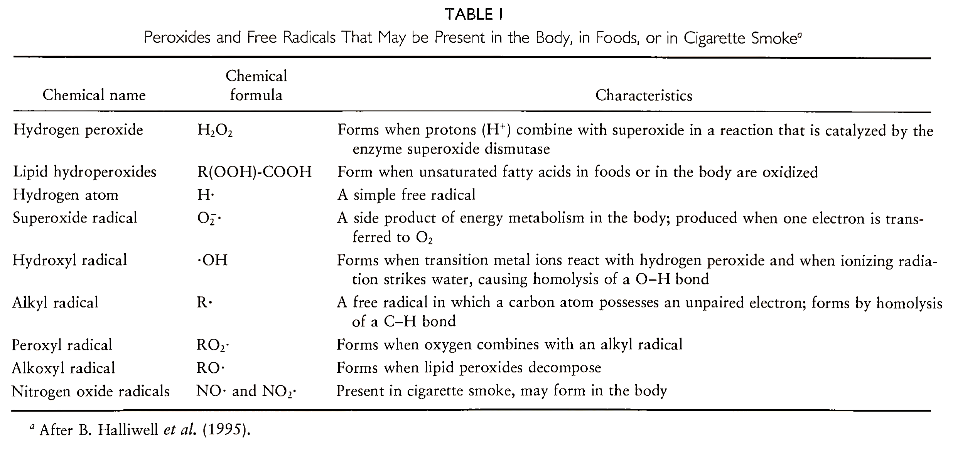
C. Reactive Oxygen Species: Nonradicals. Other reactive oxygen species important in biology are the nonradicals singlet oxygen, hydrogen peroxide, and lipid hydroperoxides. Singlet oxygen (102) has the same chemical formula as ground-state molecular oxygen but a different arrangement of electrons (it has an empty π*2p orbital but no unpaired electrons). Thus it is not a free radical but it is electrophilic because it seeks to fill its empty π*2p orbital. It reacts readily with double bonds, especially double bonds in polyunsaturated fatty acids, to produce lipid hydroperoxides:
![]()
where RH is a polyunsaturated fatty acid and ROOH is a lipid hydroperoxide. The ability of 102 to form lipid hydroperoxide is important because, as mentioned earlier, lipid hydroperoxides can decompose to free radicals.
Singlet oxygen is at a higher energy level than triplet or ground-state oxygen. It may be formed in foods and other biological systems, (e.g., skin) when light energy is captured by pigments called sensitizers. The excess energy in the sensitizer is subsequently transferred from the sensitizer to 102:
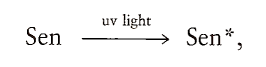
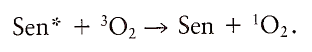
Singlet oxygen is also formed when two peroxyl radicals react:
![]()
Hydrogen peroxide (H202) may form in cells when an enzyme called superoxide dismutase (SOD) acts on superoxide anion:
![]()
Date added: 2023-01-09; views: 697;
