Trace Element Toxicants. Toxicological Evaluation. Analytical Techniques
A. Scope. Trace elements can be found in foods as natural constituents, intentional food additives, or environmental contaminants. Although Table I lists trace elements as essential or nonessential for humans, all of them can produce adverse effects if their intakes are sufficiently high. The statement by the alchemist and physician Paracelsus almost 500 years ago—“All substances are poisons…

The right dose differentiates a poison from a remedy”—is very appropriate for essential elements. Whatever method that is used for the calculation of safety limits should be based on the highest amount of the element that had been widely consumed by enough people for a long enough period of time to provide assurance that no adverse effects occur. Unfortunately, there are usually not enough clinical data for this basis, so numerous types of formulas have been used to calculate safety limits (e.g., Hathcock’s Nutrient Safety Timit, or NST).
Many of the nonessential trace elements for humans have been shown to be essential to animals, including, according to one laboratory, even toxic metals such as cadmium and tin. However, most of these data are limited, difficult to verify, and very controversial.
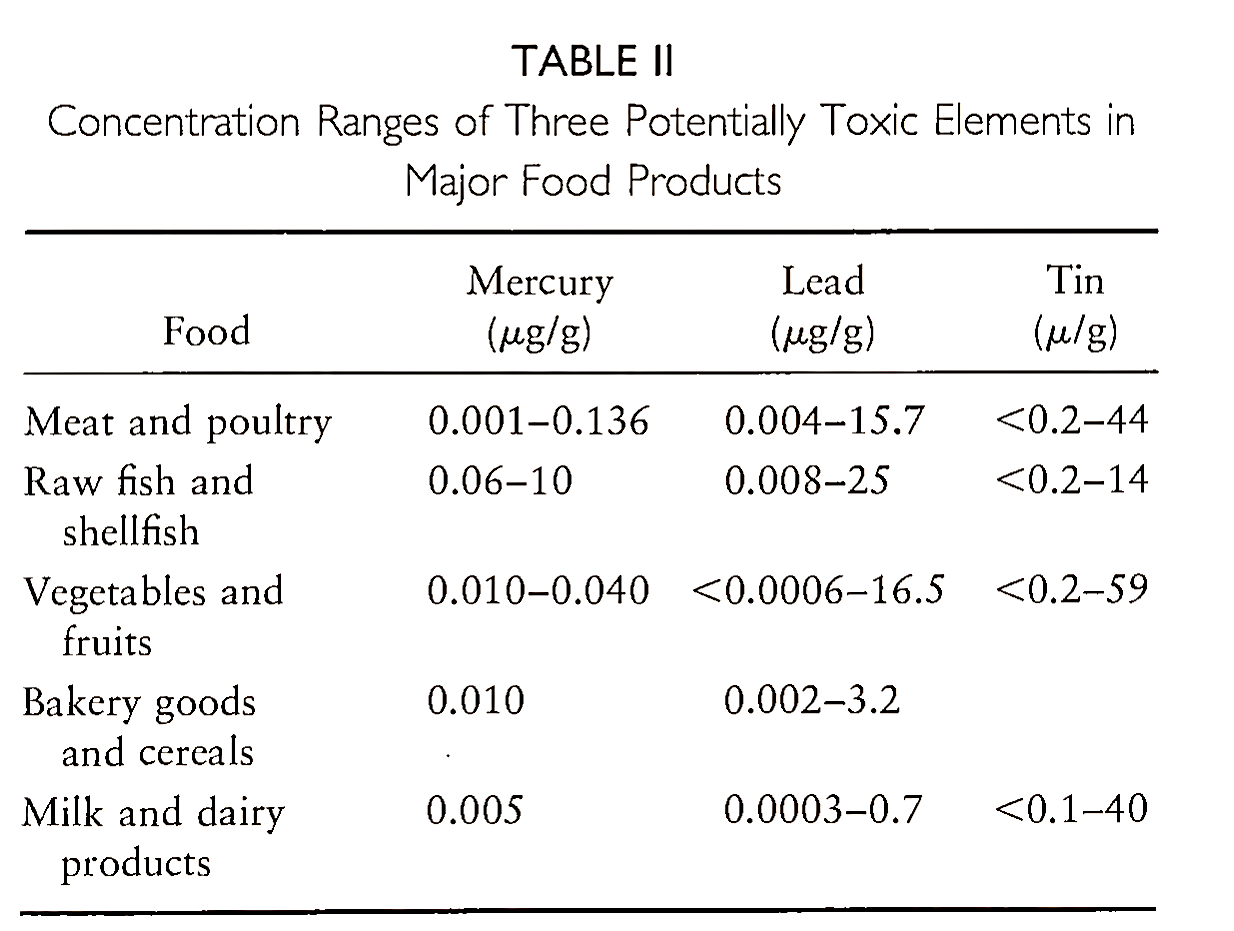
The most significant toxic effects of trace elements are mutagenicity, teratogenicity, and carcinogenicity. Numerous countries follow the FAO/WHO Codex Alimentarius Commission on tolerance limits of some of the toxic trace elements in foodstuffs, additives, and drinking water (e.g., that the daily intake of lead from all sources should not exceed 0.43 mg). Examples of the concentration ranges of three potentially toxic elements—mercury, lead, and tin—in major food products are presented in Table II.
B. Toxicological Evaluation. The amounts of trace elements in foods permit an estimate of dietary intake but do not give any information as to the element’s toxicity. This depends to a great extent on the physical and biochemical properties of the trace element species. For example, the trace element chromium (Cr) when inhaled in air as Cr(VI) is highly carcinogenic, but Cr(III) usually present in foods in very small amounts (1 ng. or 10-9 g) is an essential element (see Table I). Indeed, future legislation will refer to the maximum content for each element species rather than simply list the total amount of each element in foods.
The problem is that very little is known about the species of most trace elements in food or how processing or cooking affects or changes species; furthermore, most of the information is qualitative. In addition, almost nothing is known about the interaction of other food components with trace metal toxicity (e.g., protective factors of selenium toxicity in tuna fish).
To make an evaluation of the toxicity of a trace element, it is necessary to know its bioavailability, that is, how much is absorbed and utilized in vivo. Usually an element is not absorbed completely, but the fraction that is absorbed is dependent on the amount in the diet, the oxidation state of the element, the chemical species, and the presence of interfering or enhancing factors in the food. Unfortunately, an experimental study of trace element speciation in a complex matrix as food is a costly and difficult undertaking. In addition to detection sensitivity, there is the important need to avoid altering the speciation of the element during the various analytical steps.
C. Analytical Techniques. It is possible now to detect elements, as well as other food chemicals, at levels of picograms (10-12) per kilogram amounts by means of mass spectrometry. Concentration of the element with different oxidation states can be measured by the use of (1) electrothermal atomization-laser excited atomic fluorescence spectrometry, (2) laser-enhanced ionization spectrometry, and (3) inductively coupled plasma-mass spectrometry.
Multielement analysis is desirable to provide a scan of possible toxic levels of elements that may be present in one specific food. This analysis can enable more than one determinant to be measured in a single sample digest and greatly improves the speed of analysis. Modern instrumentation includes X-ray fluorescence, neutron activation, potentiometric stripping analyzer, proton-induced X-ray emission, and simultaneous multielement atomic absorption spectrometry with a continuum source.
Date added: 2023-01-09; views: 606;
