The chemistry of the Earth. The cosmic abundance of the elements
Present-day ideas on the chemistry of the Earth are derived from many sources because there is no direct method of sampling the interior of our planet. Data from the chemistry of objects in space (the Sun, the interstellar medium and meteorites) have been vital to the construction of current chemical models. The basic physical properties of our planet, as indicated by its moment of inertia and mass and, particularly, by the nature of seismic wave propagation, have provided critical information.
Finally any suggestions as to the chemistry of the Earth must be compatible with knowledge of the physical state of materials in the internal pressure-temperature regime of our planet. Thus any discussion of the Earth's chemistry must begin with a consideration of the chemistry of all matter in the universe.
The cosmic abundance of the elements. Analysis of the chemical composition of every type of object in the universe, including distant stars, the Sun. planets, the Moon and meteorites, is a major undertaking for scientists. The quantity of an element in a star is determined by examining the spectra of emitted radiation. Direct chemical analysis is so far possible only for meteorites and the surfaces of the Earth, the Moon and Mars. Chemical studies of rocks in the laboratory by means of highly sensitive techniques such as mass spectrometry permit analysis of all the chemical elements and their isotopes, even those present at the one part per billion level, equivalent to one gram in a thousand tonnes.
Given the basic knowledge of nucleosynthetic reactions, together with the chemical composition of the Sun, it is now possible to provide a clear interpretation of the measured cosmic abundance of the elements (Figures 4.2 and 4.3).
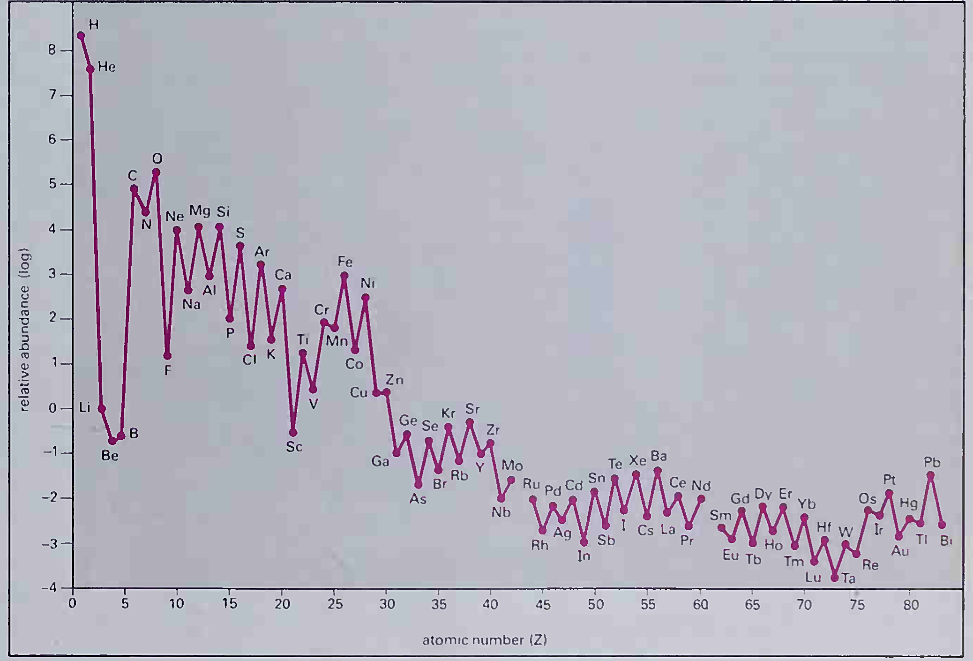
4.2 : Cosmic abundance of the chemical elements. Note the logarithmic vertical scale. Hydrogen (H) and helium (He) are about a thousand times (3 units) more plentiful titan most other elements. Abundances of all the elements have been related to that of silicon (Si I. which is given a value of 10000 (4 on the log scale). Note the pronounced peak at iron (Fe); the rarity of the light elements lithium (Li), beryllium (Be) and boron (B); and the overall oddeven effect for the abundances of neighbouring elements. H and He were produced shortly after the 'big bang'; whereas all other elements have been synthesized since by nuclear fusion reactions in the interiors of stars
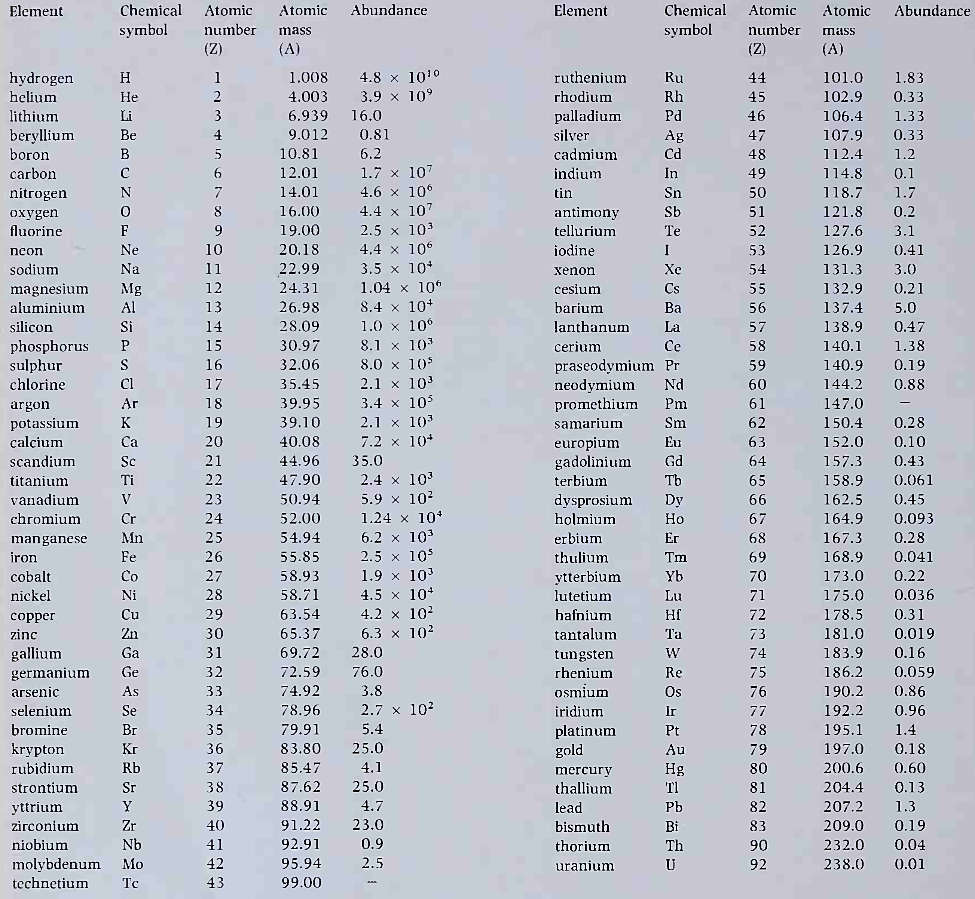
4.3: Abundances of the chemical elements in the solar system, adjusted relative to silicon, so that silicon is one million (1.0 x 106)
Hydrogen (75.4 per cent) and helium (23.1 per cent) are overwhelmingly the most plentiful elements. All the remaining elements together make up only 1.5 per cent of the total. There are eight other elements with significant abundances, including the life-forming elements carbon (C), oxygen (0), nitrogen (N) and phosphorus (P). A pronounced peak in abundance occurs for elements of atomic number close to iron, due to the maximum binding energy per nucleon. Lithium, beryllium and boron are unusually rare compared to their neighbours (helium, carbon, nitrogen and oxygen). Elements of even atomic number are more plentiful than adjacent elements of odd atomic number.
Date added: 2023-01-09; views: 800;
