The chemical composition of the Earth. The basic control of the Earth's geochemical structure
A visitor from space, approaching the planet Earth, would observe that there is a significant atmosphere, that about 70 per cent of the surface is covered by oceans and that 30 per cent of the surface is above sea level. On the basis of observations of the rocky surface, both above and below sea level, and from the increasingly sophisticated observations of modern seismology, the Earth can be regarded as consisting of a series of layers increasing in density toward the centre.
The Earth can be divided into a series of well-defined geospheres: atmosphere, hydro-sphere, crust, mantle and core. Each part may be further subdivided: the water on continents can be distinguished from that of the oceans, the continental crust from the oceanic crust, the upper and lower mantles, the outer liquid core and the inner solid core (Figure 3.12). This section summarizes knowledge of their chemistry.
Since the outer shells can be sampled directly, knowledge of their chemical composition is as good as the sampling and the methods used in chemical description. As depth increases, direct sampling rapidly becomes more difficult, and indirect approaches to ascertain chemical composition become increasingly important. Seismic data and data from cosmic chemistry, solar chemistry and meteorite chemistry-all of which provide information on the inner physical properties of the planet-must be used to obtain models of deep Earth chemistry.
The basic control of the Earth's geochemical structure. Given the composition of the various parts and their masses (Figure 4.10) an overall composition of the Earth might be like that shown in Figure 4.11. From this composition, dominated by iron, oxygen, silicon, magnesium, aluminium and the alkali metals, it is not difficult to explain the gross features of the planet. First, if all the atomic components were to be mixed in a container of appropriate volume, competition would arise between the metallic elements and non-metallic elements (O, S) to form oxides and sulphides.

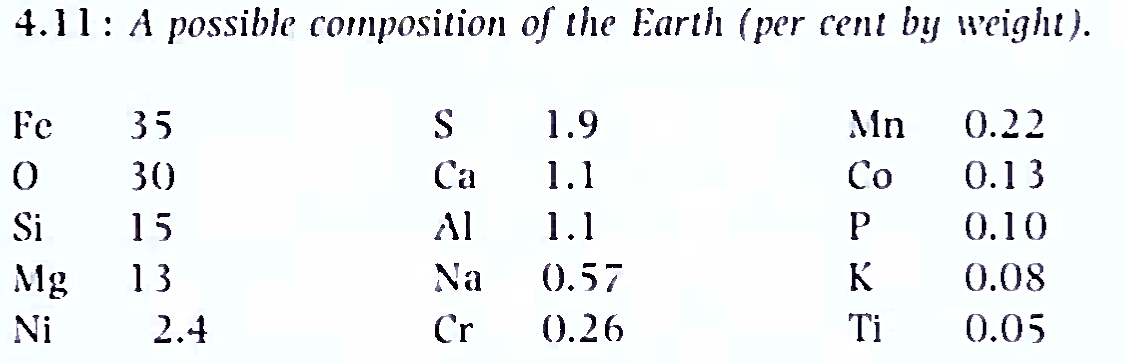
When the affinity of the elements with oxygen is considered (based on the free energy of formation of the oxides) it is clear that species such as MgO, Si02 . Al2O3. Na2O and CaO would form in preference to FeO. Thus as the bulk composition is oxygen-deficient, most of the iron and nickel would be left in the unoxidized state. Of course, the separate oxide phases would combine to form silicates (eg MgO + Si02 MgSi03 (pyroxene) or olivine (Mg2Si04)).
If the condition of gravitational equilibrium were now to be imposed the most dense materials would be at the centre and the mixture would stratify, forming tlic dense inner metallic core and the outer silicate mantle. Atomic species at low concentrations would tend to separate in a sympathetic manner. Thus a noble metal such as gold would tend to be associated with metallic iron, and an oxyphile element such as uranium with the lighter silicate fraction.
Next it may be assumed that the Earth is heated until it is totally or partially molten. The source of this energy maybe energy of accumulation, energy of core separation or radioactive heating. Given such heating and partial fusion, volatile species with very low melting temperatures (H20. C02, N2, Ar etc) will rapidly boil off, to form the atmosphere and hydrosphere systems. In the mantle the most fusible light liquids will rise to the surface, enriching the upper layers, in such components as Si02. AL2O3 . Na2 and K20, while the heavy fusible components will move to the core (Fe. Ni, FeS etc).
Early geochemists recognized this basic pattern of separation of the elements and classified elements as atmophile (preferring to be in the atmosphere), lithophile (in silicates and oxides), chalcophile (in sulphides) and siderophile (in iron or metals).
Given a hot early Earth rapid separation into the layers described above would be expected. The separation is essentially a result of the volatility, fusibility and density of the most stable chemical units (metals, oxides, silicates etc). However, it should be noted that, given the very high pressures and temperatures within the Earth, compounds may form in structures quite different from those that are familiar at the Earth's surface. Thus while Si02 occurs at the surface as the common mineral quartz, of density 2650 kg/m3 . at depth the common mineral will be the polymorph stishovite of density 4290 kg/m3. All present evidence shows that the Earth had reached some-thing like its present structure about 4000 million years ago. The oldest samples of crust, dating from about 3800 million years ago. contain rocks quite similar to those forming today, and most were formed in a submarine environment.
Date added: 2023-01-09; views: 778;
