The present geochemical cycle
Over the past few decades there has been a spectacular accumulation of evidence to show that the Earth is cooling by a convective as well as by a conductive process. The convective process controls most of the major surface processes, such as continental drift, ocean floor spread ing, mountain building and volcanism. Convection is a mixing process and, in recent years, geochemists have become aware that this phenomenon must be considered when models of Earth chemistry are posed.
Not all the aspects of the convective process have yet been quantified. This section discusses general chemical processes associated with present Earth dynamics, for a knowledge of present processes is vital to our understanding of those of the past. Ultimately models of the distribution of elements must take into account these cooling and mixing processes. Clearly the present distribution of elements in the major layers of the Earth has not necessarily always existed.
The geochemical cycle, Stage 1: rising convection cells Radioactive heat production in the mantle leads to the formation of hot mantle plumes, which rise at widely spaced centres to produce the volcanic phenomena observed at the great ocean ridge mountain ranges. At these ridges new light crust is formed from magmas, which crystallize to rocks of the basalt-peridotite group. This new crust forms at a rate of about 4 x 1013 kg/a. and the process carries almost half the energy produced in the interior to these highly localized sites. Most of the phenomena occur in a submarine environment. The new crust is formed, from both extrusive volcanic rocks and their intrusive equivalents, beneath the lava cover. The process is rapid, and has formed about 70 per cent of the Earth's crust in the past 200 million years, ie 5 per cent of the Earth's history.
Most of the new magmas arriving at or near the surface cool and crystallize in a submarine environment. As magma cools it contracts, cracks and becomes porous and permeable. It has been directly observed and shown by all theoretical treatments that, where there are hot permeable rocks under seawater. cooling must involve convective circulation of the latter. At the ocean ridges almost half the thermal energy in the new hot crust is transferred to seawater. Observers in submersibles have recorded impressive discharges of hot water near submarine volcanic sites, with hot water reaching temperatures in excess of 300 C.
The interaction of cold seawater with hot basaltic rocks produces profound chemical changes in the rocks and modifies the seawater. Thus an exchange process occurs between the hydrosphere and new mantle-derived crust. The scale of the exchange is related to the thermal energy available at the ridges, and the entire ocean mass is circulated through the ridge zones every few million years.
Recent studies of ocean floor rocks, both in situ and where they appear on land (notable occurrences can be found in Cyprus and Oman), show the profound chemical changes that occur. For example H2 is fixed in hydrated minerals: 0, dissolved in seawater oxidizes the upper layers and produces minerals such as haematite and magnetite (Fe203 and Fe204 l: bicarbonate in ocean water is fixed in carbonate minerals: sulphate in seawater may be precipitated as calcium sulphate, or reduced to form pyrite (FeS,) by reaction with iron in the basalts; sodium ions in seawater exchange with cations in basalt silicates to form the mineral albite (NaAlSi308 ): and potassium ions in seawater are fixed in complex clay minerals formed by low temperature alteration.
As the cold seawater convects down into the basaltic crust, it becomes heated, and eventually discharges back to the surface. On account of the exchange reactions the fluid discharged is quite different in chemistry. It becomes enriched in gaseous hydrogen and especially in trace metals, resulting from the sodium-potassium fixation processes. Almost all the transition metals are enriched, and metals, present as chlorides, are returned to the ocean—rock interface: they include Cu. Mn. Fe. Cr. Ni. Zn. Ag and Au. Some of these metals may be precipitated as the ascending fluids cool, some may be precipitated as sulphides at the marine interface and some may be dispersed into the overlying seawater to contribute to metal-rich muddy sediments.
Such fluids may also contribute to the elements that form the widely distributed manganese nodules rich in Mn. Fe. Co. Ni and Cu. At the present time these phenomena are the subject of intensive research, and already submersibles have directly observed and sampled new metallic ores formed by such processes. The altered rocks are chemically complex, as is to be expected for a convective process, where the pressure, temperature and chemistry of the fluid all change along the flow path.
At the great ocean ridge systems of our planet new crust is formed as a product of partial fusion of rising hot mantle. This rock is modified by cooling, through seawater exchange. Components from the atmosphere and hydrosphere are added to the solid crust, and the oceans are enriched in some components from the mantle. Many of the great sulphide and oxide ore deposits are formed by this process.

4.16: A manganese nodule dredged from the floor of the Pacific Ocean. The maximum diameter is 70 mm. The average composition of the nodules is 20 per cent manganese. 15 per cent iron, varying amounts of clay, calcium carbonate, and volcanic fragments, and traces of copper, cobalt and nickel. This material is built up by accretion around a nucleus, which can be a pebble, volcanic fragment, organic particle, or even a shark's tooth
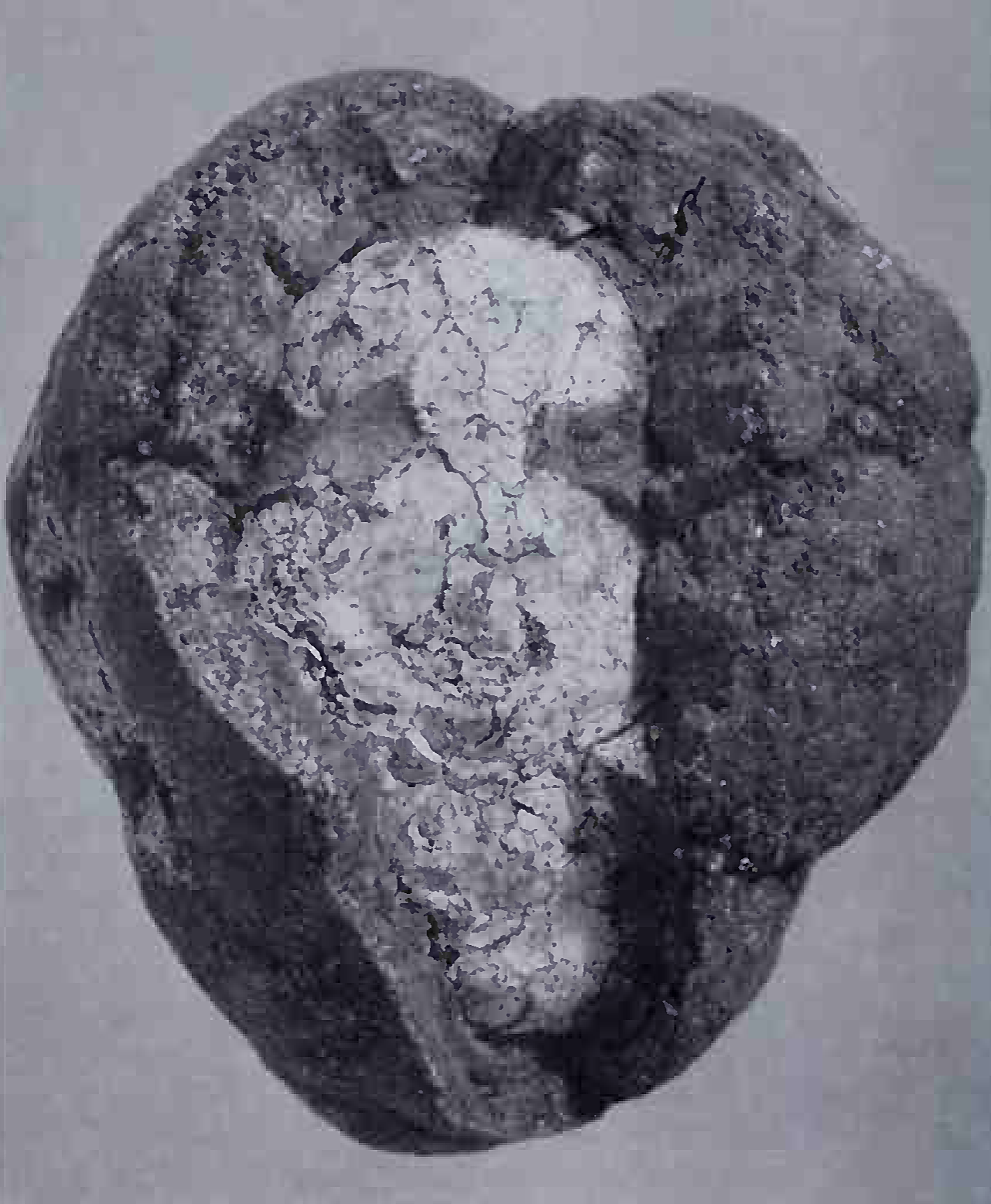
4.17: A broken surface through the same nodule displaying the characteristic internal layering which is built up by accretion at a rate in the order of 1 mm per 5 million years
Date added: 2023-01-09; views: 609;
