Earth materials: minerals and rocks. The physical properties of minerals
Minerals are the basic chemical compounds of the solid Earth. About two thousand minerals are known, and between them they contain all the chemical elements of which the Earth is made. Rocks are physical mixtures of minerals, usually in the form of interlocking crystals or of grains bound together by a natural cement. An enormous variety of rocks make up the Earth, especially its outer crust (about 18 to 50 km thick), but the vast majority are composed of not more than a few dozen of the two thousand minerals that are known.

5.2: (a) Crystals of pyrite (FeS2) showing features of cubic symmetry
The physical properties of minerals. Nearly all minerals are crystalline with compositions that are fixed or vary within specified limits. This means that they have well-defined physical and chemical properties which give each mineral a separate identity. Under appropriate physical and chemical conditions minerals can develop crystal forms that are among the most beautiful of natural shapes. Well-crystallized minerals are bounded by regular arrangements of plane surfaces, called crystal faces, which are characteristic for each mineral and independent of size.
All crystals possess symmetry, though it is not always immediately apparent. The cubes of pyrite and the hexagonal columns of quartz in Figure 5.2 are obvious enough, but the symmetry of others is more elusive. The underlying symmetry of a crystal can be further obscured when the sizes and shapes of its faces are not uniform, as frequently happens.

The quartz crystals in Figure 5.2, for example, all have both columnar and pyramidal faces, but the latter have developed differently in each crystal. However, the angles between the columnar faces (and the pyramidal faces) are the same in these crystals and on all other quartz crystals, wherever they occur (Figure 5.3).
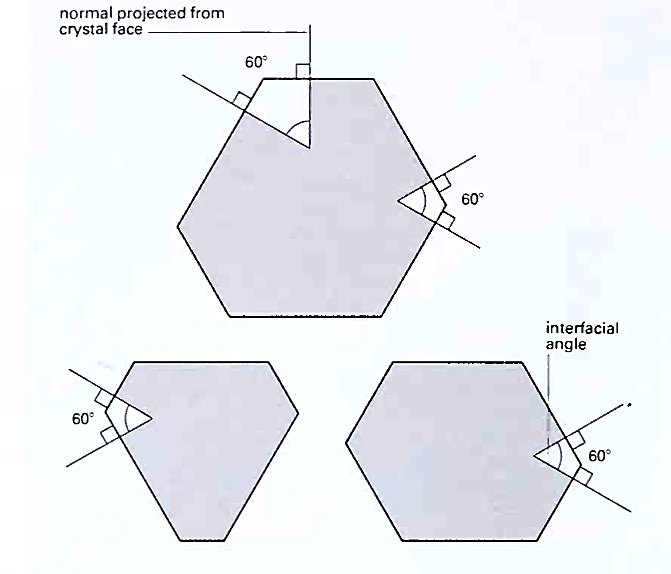
5.3: Diagrammatic cross-sections through three quartz crystals showing how the angles between the columnar faces are always the same. Interfacial angles are conventionally defined as those between the normals to the faces (60c in this case)
The constancy of interfacial angles, which underlies the patterns of symmetry among crystals, was first established by Nicolaus Steno (1638-86), a Danish monk, in the seventeenth century. Systematic measurements of interfacial angles on countless specimens of both natural and artificial crystals have enabled mineralogists to establish that all crystalline substances can be assigned to one of seven symmetry systems, each characterized by specific combinations of symmetry elements of which the principal ones are axes, planes and centres of symmetry.
Crystals of the same mineral can vary widely in appearance because crystal faces develop in different ways depending upon the conditions of crystallization. The common mineral calcite is a good example, and two of its numerous crystal forms are portrayed in Figure 5.6(a).

A great many minerals break along planes of weakness called cleavage planes, so that broken fragments are bounded by more or less well-developed plane surfaces. These cleavage surfaces are sometimes so smooth that they can be mistaken for crystal faces. The direction of cleavage in minerals is commonly parallel to crystal faces, though equally commonly it is not. Cleavage planes bear precise geometrical relationships to one another as well as to crystal faces, and these relationships are characteristic for each mineral: intercleavage angles are constant, just as interfacial angles are.
A particular mineral may develop several different combinations of crystal faces, depending on the environment of crystallization, but the number and orientation of its cleavage planes are always the same. Cleavages represent planes of weakness in the mineral. Crystal faces are planes of growth.
Crystal growth is rarely uniform; crystal faces frequently develop unevenly, and some minerals have a tendencyto form twinned crystals. In these a crystal face becomes a twin plane, on either side of which the crystal develops in a different orientation, forming in effect two (or more) crystals joined together in a definite geometrical relationship.
In the most straightforward type of twinning two halves of a crystal are mirror images of each other, but more complex twins involve relative rotations of individuals on either side of the twin plane. Some minerals are more prone to twinning than others, and some of these develop multiple twins, made up of several twinned subindividuals within a single crystal.
Date added: 2023-01-09; views: 706;
