The atmosphere. The hydrosphere. The crust. The mantle
The atmosphere of Earth is unique in the solar system. At sea level atmospheric pressure is about 1 atmosphere, ie 1 x 105 pascals (Pa). The total mass of the atmosphere is 5.3 x 108 kg (see Figure 4.10), a very small part of the total mass of the planet, which is 5.97 3 x 1024 kg. The nature of the atmosphere is a result of gravitational stratification and the very complex photochemical interactions that take place between solar radiation and the molecular species present.
At sea level the typical composition is as shown in Figure 4.12. Except for N2. O2, and Ar. all gases are present in very small amounts. The thermal structure of the atmosphere is a complex result of pressure change, radiative balances and photochemical processes. Thus temperatures decrease upwards from the surface to about 12 km increase from 12 to 50 km. decrease again to about 100 km and then steadily increase.
At high altitudes photodissociation processes are extreme, and single atoms or ionized atoms dominate the tenuous atmosphere: in the upper layers of the atmosphere a large fraction of light atoms may reach the escape velocity, and it is estimated that about two-thirds of all hydrogen atoms present will escape in about a thousand years. There may also be some loss of helium, but for all heavier species escape is negligible. As all hydrogen compounds are subject to photochemical dissociation, their lifetime in the upper atmosphere is short.
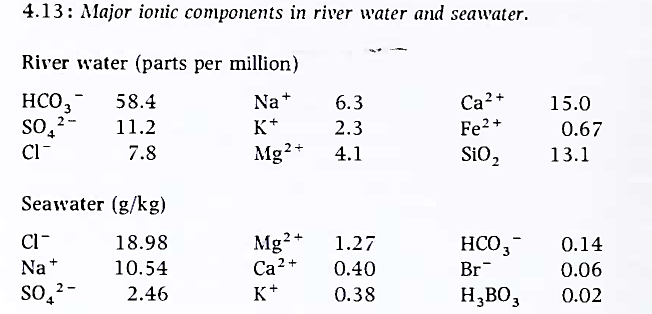
The hydrosphere. The mass of liquid water on our planet is about 1.4 x 1021 kg. about 2 per cent of the mass being in streams, lakes and ice sheets, while 98 per cent is in the oceans. Continental (fresh) waters are relatively dilute solutions, while ocean waters are more saline (Figure 4.13). Inspection of the appropriate data shows that ocean water does not simply result from concentration of inflowing river waters, but that interactions with the solid crust must play a major part in its chemical evolution.
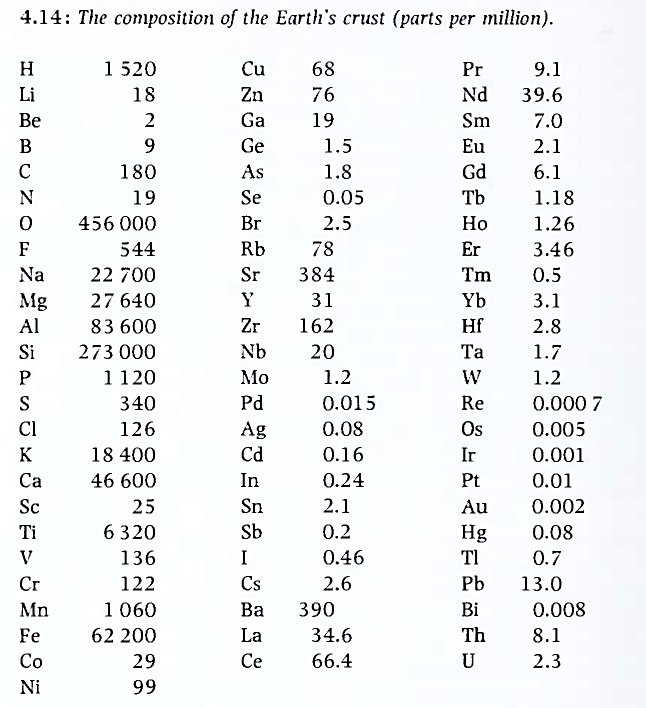
The crust. The outer layers of the solid Earth, the crust, consist mainly of materials with density below 3000 kg m3. This crust can be divided into continental crust (mass 1.6 x 1022 kg), with an average density around 2700 kg/m3. and ocean floor crust (mass 7.0 x 1021 kg), with density around 3000 kg/m3. The continental crust averages about 30 km in thickness, while the oceanic crust is in the range of 5 to 10 km in thickness. Knowledge of the chemical composition of the crust results from sampling the various rock types accessible at the surface, and from mining and drilling beneath the surface. Only recently has ship-based drilling made it possible to sample the ocean floor crust. The rock types most likely to be dominant at greater depth must be assessed from knowledge of structural geology or from seismic studies. Clearly, there are many uncertainties in deciding on the average composition given in Figure 4.14.
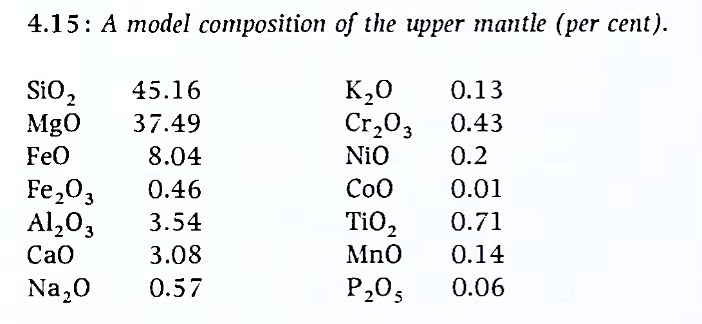
The mantle. It is known from seismic studies that the mantle, extending from 10 to 90 km beneath the surface, to the core boundary at 3486 km from the Earth's centre, is made of materials considerably more dense than those of the crust. This region of the Earth cannot be sampled directly and, as noted above, ideas on its chemistry are based on cosmic chemistry, meteorite chemistry, seismic properties etc. Further constraints are imposed by what is known of the overall heat production of the Earth and, of great importance, by the fact that the material must be capable of producing the volcanic magmas that are known to rise from the deep interior at oceanic ridges and volcanic islands such as Hawaii. Knowledge of the chemistry of the mantle, particularly in its deepest parts, is uncertain.
The typical mantle composition shown in Figure 4.15 will allow production of basalt liquids and will produce mineral phases of appropriate seismic properties at depth. Modern evidence of element and isotopic abundances clearly shows that the mantle is not uniform in composition. The abundances of siderophile elements such as Pt, Pd and Au indicate that these must have been largely partitioned into the metallic core during core formation.
The core. Seismic studies and the total mass of the Earth clearly show that the core (total mass 1.9 x 1024 kg, radius 3485.7 km) must have an average density of 11 000 kg/m3. The inner core is solid while the outer core is liquid. Further, the core must have properties appropriate to the generation of the magnetic field. Given the density constraints, and the seismic constraint that the mean atomic number of the core is a little lower than for pure iron, most researchers assume that the core is dominated by iron and nickel (as in some metallic meteorites), but with some dilution by light elements.
Candidates for this dilution include C, S, Si, H and perhaps O. At present the exact composition is still a matter of debate. As discussed in the paragraph above, the core probably contains most of the Earth's siderophile elements.
The biosphere. While the total mass of living matter is small (1015 kg), the biomass is important in concentrating and transporting certain species in the hydrosphere-atmosphere system. Most biological species require a wide range of elements, and concentrations in living organisms can lead to remarkable enrichment of some metals.
These processes are often reflected in the metal content of fossil carbon deposits, such as coal. Thus there are elements that can be considered as biophile. Biological activity is very important near the surface, for it can produce highly reducing conditions, eg in subaqueous environments or, in the regions of photosynthesis, oxidizing conditions.
Date added: 2023-01-09; views: 651;
