The origin and distribution of the elements
An important goal of astrophysics, and of fundamental importance to the Earth sciences, is to account for the origin and distribution of the chemical elements. Simply discovering the present composition of the Earth, the planets, the Sun. stars and the Galaxy is a major occupation.
The actual distribution of the chemical elements in the universe can be found either by the direct analysis of samples in the laboratory or by the detailed analysis of the spectra of distant bodies.
The former method can be applied at present only to the Earth, the Moon and meteorites. All the remaining data on the relative abundances of the elements have come from spectroscopy. The optical spectra of the Sun and stars have in them thousands of dark absorption lines which are caused by the various atoms in the cool upper atmospheres of the stars. The relative strengths of these lines can be measured and interpreted to yield the relative abundances of the various elements in a particular star (Figure 2.10).
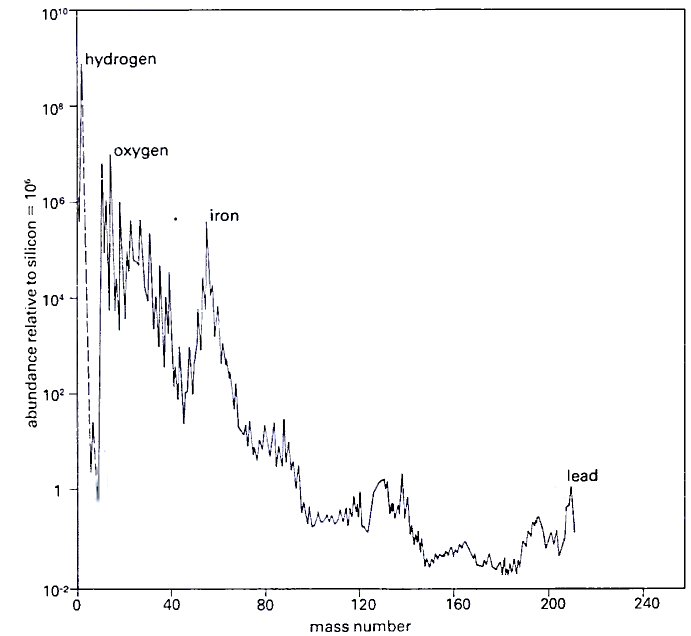
2.10: The relative abundances of the elements in the solar system plotted against atomic mass. Astrophysicists have shown how the main features of this curve arise. All elements other than hydrogen and helium have been manufactured in cosmic nuclear furnaces and explosions
It is clearly the case that the inner planets of our solar system, such as Mercury and the Earth, have very little of the lightest and most volatile elements left ; after all. they are rocky planets. The outer giant planets, such as Jupiter and Saturn, are probably nearer to possessing the original composition of the solar system. The composition of the outer layers of the Sun itself is probably the best clue to the nature of the material from which the solar system condensed ; the Sun has a strong gravitational field and it cannot have lost its lighter gases selectively in the way that the Earth has.
The outer layers have not been contaminated by the helium manufactured at the centre. The solar abundances show that, by mass, its composition is approximately three-quarters hydrogen and one-quarter helium, a faithful reflection of the elemental distribution in the early universe. This is not the whole story, however: about 1 per cent of the Sun's mass is contributed by the so-called heavier elements, that is elements other than hydrogen and helium. The relative abundances of these elements show some interesting trends.
There is a general decrease in abundance as we go to heavier elements: lead and gold are far scarcer cosmically than silicon and sulphure, and a handful of the lightest elements, lithium, beryllium and boron, are rare relative to their neighbours in the periodic table of the elements. These light elements are destroyed early in the evolution of protostars. There is also a bunching of abundant elements around iron.
The general features of the Sun's composition today can be taken to be typical of the material from which the planetary system formed. It would be unreasonable to suppose that the planets condensed from cloud fragments with a fundamentally different composition to the proto-Sun. It is also reassuring that the relative abundances of the heavier elements in the Earth's crust, the lunar rocks and meteorites, are entirely consistent with the solar abundance distribution.
Most stars have a composition similar to that of the Sun. if exotic kinds such as neutron stars are excluded. It can, therefore, be taken that our Sun and solar system are not the products of any rare astronomical circumstances.
It used to be supposed that the universe has always had its present chemical composition, but it is now clear that the products of stellar evolution, and especially of supernovae, have modified this assumption. Astrophysicists are in reasonable agreement that the cosmic ubiquity of hydrogen and helium indicates that these at least are primeval, and their relative proportions were essentially fixed by the time the universe was ten minutes old.
The remaining elements have been synthesized subsequently, by controlled and explosive burning in stars. Astrophysical theory is able to give a reasonable explanation for the abundances that are actually seen. It can explain why iron-group elements are common; why the post-iron-group elements (those of higher atomic number than iron) are rare; why lithium, beryllium and boron are scarce; and why nuclei and isotopes that have a nucleon number that is an exact multiple of four are commoner than those that are not exactly divisible by four. These successes indicate that astronomers have been able to give a coherent account of the history of matter.
It can now begin to be understood why the Sun and the Earth have arrived on the astronomical stage relatively late. The Earth is overwhelmingly composed of heavier elements, those that simply did not exist in the early universe. It was not until earlier generations of stars had exploded as supernovae. contaminating the interstellar medium with the products of nuclear burning, that stars with heavier elements could be born.
Out of these cosmically enriched clouds smaller bodies containing elements other than hydrogen and helium could condense also. Practically all the nuclei in all the atoms on the Earth (except the hydrogen in the water) have been manufactured in massive stars that were born, lived and exploded long before the Earth itself could form. The carbon nuclei in the black ink on this page were cooked up ten billion years ago in a stellar furnace with a temperature of 108 degrees centigrade.
Date added: 2022-12-12; views: 714;
