Landfill Leachate Composition and Interaction with Groundwater
Leachates from landfills have a wide range of compositions and chemical parameters. The review by Christensen et al. provides a diverse list of components including inorganic macrocomponents, trace metals, and organic compounds.
A selected list of these components in landfill leachates is presented in Table 1. In addition, a series of chemical parameters is also presented for the two main stages of leachate evolutionary stage, as presented in the previous section (Table 2).
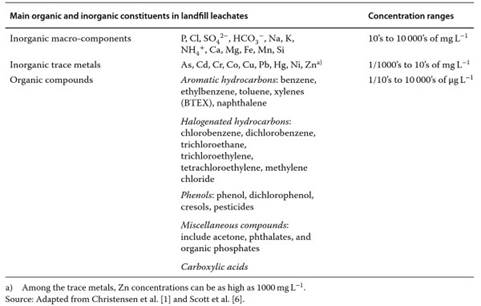
Table 1. Selected list ofinorganic and organic constituents of landfill leachates as compiled from,, which can be consulted for detailed concentrations and sources
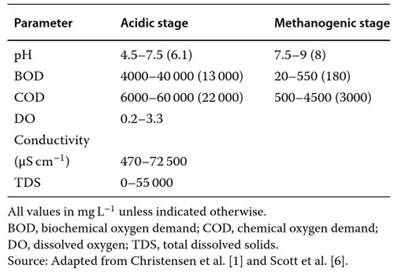
Table 2. Range ofvalues and averages (in parenthesis)ofselected parameters for landfill leachates in the acidic and methanogenic stages, unless unspecified
These parameters, species, chemical compounds, and trace metals tend to vary widely depending on landfill type and specific materials disposed at these facilities. In either case, episodic spilling and leaking of the leachate into groundwaters is subject to monitoring and facilities are constructed to prevent such environmental risks. However, spilling of leachat may happen for several reasons, and in that case, it is important to understand what happen to the organic compounds and associated inorganic constituents, including trace metals (Figure 1).
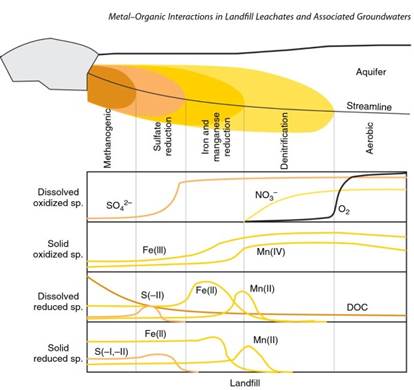
Figure 1. Landfill leachate plume dispersion in an aquifer with the development of the different redox fronts. The graphics below provide a qualitative representation of the concentration variations of the solid/dissolved reduced and oxidized species in the plume domains. DOC, dissolved organic carbon. Source: Adapted from Christensen et al.
As a highly reductive solution, organic compounds in leachates tend to be gradually decomposed in groundwaters by means of a set of redox reactions with increasing energy yield as methanogenesis > sulfate reduction > iron reduction > manganese reduction > nitrification > aerobic respiration (Figure 1).
This sequence of reactions, which depend on the availability of redox species in the aquifer matrix, controls the dynamics of plume dispersion and has several consequences on trace metal solubility, as many of the precipitated solid phases can be important metal scavengers, such as oxides and sulfides. Therefore, metals persisting in organic-rich solutions will interact differently with organic molecules, a subject that will be discussed briefly.
Metal-Organic Complex Stability. Metals are not the most important component oflandfill waters. However, because they are persistent pollutants in the environment, knowing their fate is of utmost importance. Toxicity of metals to living creatures is a well-known issue that in many circumstances is dependent on abundance because several metals are essential nutrients at trace concentrations.
Despite such risk, not every chemical form is equally hazardous, one such example is Cr, being an essential component to glucose metabolism, it is also extremely poisonous in the chromate form (Cr(VI)). On the contrary, Cr(III) is not particularly toxic and forms insoluble solids, which reduces its bioavailability.
Depending on the speciation and complex formation, metal cations may have different migration velocities in groundwater, which can change with metal-organic ligand association, and eventually reduce their bioavailability.
Metal-organic interaction in landfill-contaminated waters depends on the stability of complex formation and metal concentration in solution. Classification of metals into hard-sphere (type A) or soft-sphere (type B) cations is helpful in grouping them according to type of complex formation and stability. Hard-sphere cations have noble gas configurations with spherical symmetric orbitals, because of the low polarizability of the electron cloud.
These cations form ionic bonds mostly with ligands that have oxygen as a donor atom. Alkali and earth-alkali metals and Al3+ are examples in this group. Soft-sphere cations have deformable electron clouds because of their greater polarizability. These metals predominantly form covalent bonds with ligands having S or N as donor atoms. Metal cations in this group include Cu+, Zn2+, Cd2+, Hg2+, Pb2+, Sn2+, and Tl3+. Besides, transition metal cations, such as V2+, Cr2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, V3+, Cr3+, Mn3+, Fe3+, and Co3+, also have similar properties.
For transition metal cations, a reasonably well- established rule for a sequence of complex stability indicates that the stability increases in the series (known as the Irving-Williams order) Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ > Zn2+ [7]. For hard-sphere cations, complex stability is proportional to the charge/radius ratio of the cation.
Complexes with monodentate ligands are usually less stable than those with multidentate complexes (chelates). Normally, monodentate complexes are easily dissociated at dilute concentrations of the metal cation, which is well illustrated in experiments with monodentate, bidentate, and tetradentate Cu(II) amine complexes. At dilute metal concentrations (<10-4M), where the monodentate complex is fully dissociated, the tetradentate complex remains stable in solution.
Thus, even at concentration levels that are likely to be found in leachate-contaminated waters, the complex- ing effect of monodentate ligands is expected to be negligible. This experimental evidence may justify the verification that the highly abundant monocarboxylic acids on leachate-derived waters had little influence on the adsorption of metals such as Cu and Zn onto solid phases.
Schilling and Cooper identified that carboxylic groups are the most important binding sites for Cu(II) cations in soil organic matter and that phenol groups are less important. Increase on metal sorption capacity is also observed with the addition of carboxyl and amino groups to various types of biomass.
Date added: 2023-10-03; views: 766;
