Thermodynamics of Freshwater Chemistry
Introduction. Many of the articles in this Encyclopedia focus specifically on various aspects of water chemistry. Other articles draw on concepts of water chemistry related to, for example, ecotoxicology, microbial processes, or mineral growth and dissolution. As background for the many discussions of water chemistry, a basic review of some key concepts, equations, terms, and approaches in environmental hydro-bio-geo-chemistry is provided here.
This article deals specifically with thermodynamic considerations, focusing on freshwater systems. The material presented here is drawn largely from author Maurice’s book Environmental Surfaces and Interfaces from the Nanoscale to the Global Scale.
This article begins by introducing some commonly used chemical units. Next, the difference between thermodynamic and kinetic approaches is discussed briefly. This is followed by the discussion of some fundamental thermodynamic theory, such as the difference between concentration and “effective" concentration or activity. Finally, some background on carbonate and redox chemistry is provided, along with some notes on aqueous geochemical computer models.
Elsewhere in the Encyclopedia, readers can find closely related articles such as Acid-Base Chemistry of Water and Carbon Dioxide and Carbonate Chemistry of the Oceans. A host of other articles deal with organics, nutrients, rare earth elements, nanoparticles in water, geochemical modeling, and many other important components and issues related to water chemistry.
Concentration Units. Water chemists generally speak in terms of different concentration units. These units are needed to perform calculations involving the chemical compositions of natural waters. Some of the most important units include (wt = weight) the following:
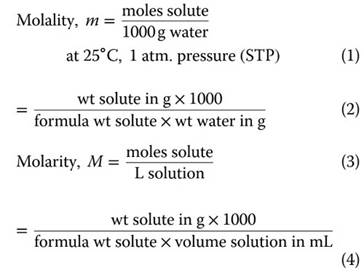
For dilute aqueous solutions at standard temperature and pressure (STP), m generally = M. However, this is not true for highly concentrated aqueous solutions, particularly in saline environments.
Another common chemical unit, equiv L-1, takes into consideration the charge on an ion. To convert from M into equiv L-1, we simply multiply by the absolute value of the charge on the ion, z:
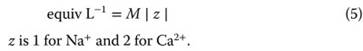
Mole fraction, Ni, is the ratio of the number of moles (n) of the given constituent i (ni) to the total moles of all constituents j,
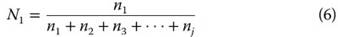
Mole fractions are most commonly used for describing minerals rather than water per se, but are important for various water-rock interactions such as adsorption to minerals and mineral dissolution or weathering.
Other commonly used units include parts per million (ppm) and parts per billion (ppb):

Thermodynamic Versus Kinetic Approaches. In order to understand the difference between kinetic and thermodynamic approaches to studying water chemistry, we can begin by considering a change in the state of a hypothetical system from state A to state B (Figure 1). This system is closed or isolated from its environment. In Figure 1, the system at state A changes or reacts to form a second state B according to the overall reaction:
A ↔ B (9)
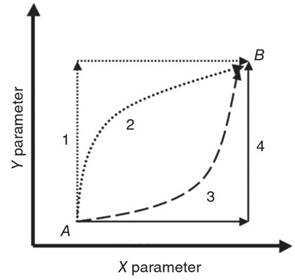
Figure 1. A schematic illustration of a change in a system from state A to state B. The x- and y-axes may represent changes in temperature and pressure, respectively, or some other parameters. For example, the state changes could be from a solid to a liquid or from a certain mineral assemblage to another
Chemical thermodynamics describes the overall energy change that occurs upon changing from state A to state B, which is independent of the pathway taken (i.e. independent of the detailed set of reactions or processes). Chemical thermodynamics is the study of energy and its transformations. Chemical kinetics, in contrast, is the study of rates and mechanisms of reactions.
Chemical kinetics focuses on the detailed rates and mechanisms involved in going from state A to state B along different potential pathways. Each pathway may consist of a different series of reactions or processes. Chemical thermodynamics tells us that the overall energy of the change from state A to B is independent of the pathway taken. Kinetics focuses on the individual pathways, including the time dependency of reactions and the detailed reaction sequences.
Date added: 2023-10-03; views: 660;
