Radiant Energy and the Ocean
The ocean's source of radiant energy is the sun. The sun emits a spectrum of radiation frequencies, of which 50 percent are infrared, 41 percent visible light, and 9 percent ultraviolet, X rays, and gamma rays. The frequencies denote sizes of quanta of energy (called photons). As solar radiation penetrates first the atmosphere and then the ocean, quanta that excite the molecules in air or in water are absorbed.
Figure 6-7 shows that an infrared photon is absorbed poorly in molecules in air but quite well in water molecules; that ultraviolet photons are absorbed best by air molecules; and that blue and green photons are least absorbed in both air and water. Therefore, infrared radiation passes through the atmosphere (the gases in the atmosphere, oxygen and nitrogen, are transparent to infrared radiation) but is absorbed by water.
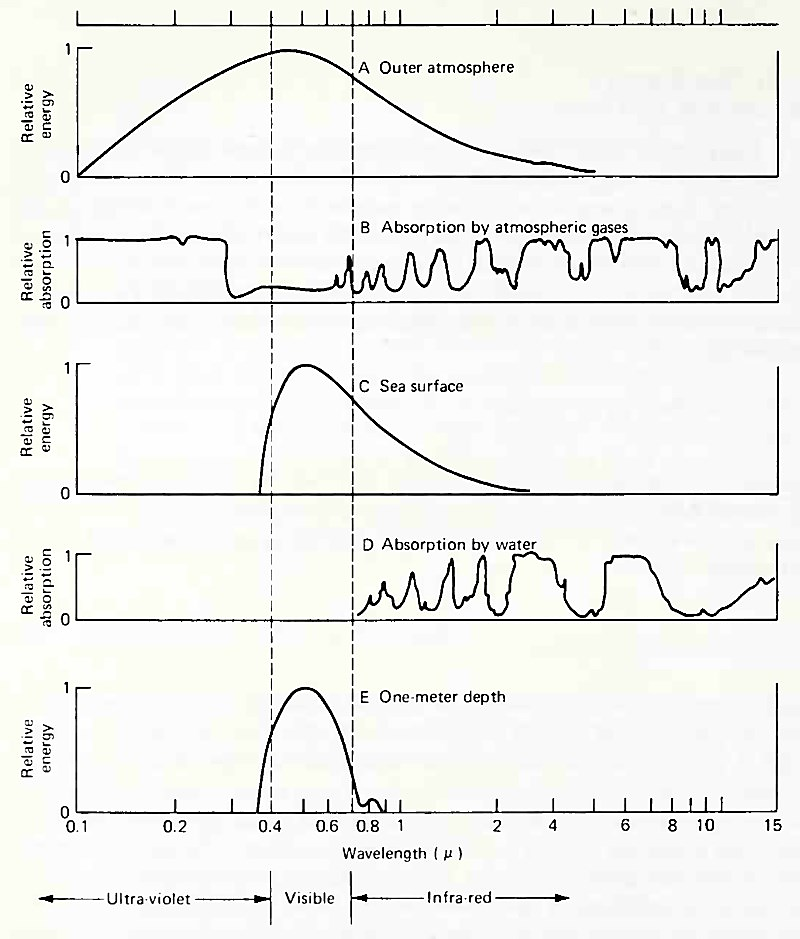
Figure 6-7. Relative change in solar energy due to absorption as it passes through the earth's atmosphere and penetrates through one m of seawater. (A) Energy spectrum of solar radiation reaching the outer atmosphere; (B) Absorption due to the principal absorbing gases in the atmosphere; (C) Energy spectrum of solar radiation reaching the sea surface; (D) Absorption by seawater; (E) Energy spectrum of solar radiation reaching one m depth. (Adapted in part from Miller, Merrill Books Inc., and Sverdrup et al., Prentice-Hall, Inc.)
Because infrared photons and some visible light quanta are absorbed into molecules of water in the same way as thermal energy quanta, either infrared photons or thermal energy excites the water molecules. As a result, absorption of infrared photons increases the heat energy and the temperature of the water.
The wavelength of radiant energy emitted by a body is proportional to its temperature, so the earth emits radiation that has its peak wavelength in the infrared. Infrared photons are strongly absorbed by water molecules; consequently, much of the radiant energy emitted from the ocean (and land) is absorbed by water vapor or clouds in the atmosphere. Fig. 6-8 compares the incident radiant spectrum emitted by the sun (temperature = 5,700°C) and absorbed by the ocean with the spectrum emitted by the ocean at 20°C.
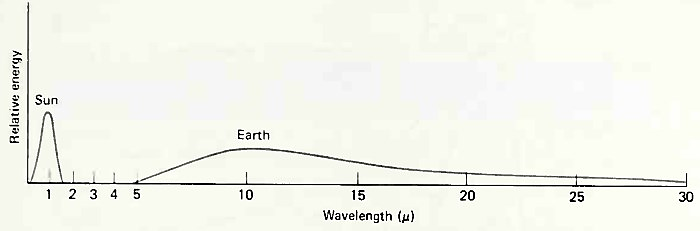
Figure 6-8. Comparison of emitted radiation of the sun and earth
Infrared radiation is so strongly absorbed by water that the incident solar infrared (0.8 to 2.5 μ) radiation does not penetrate the ocean deeper than about 1 m. Consequently, solar heating occurs in only the upper few meters of the world ocean, and heat energy is carried deeper only by conduction and vertical mixing. The general picture of the distribution of temperatures in the ocean is shown in Fig. 6-9.
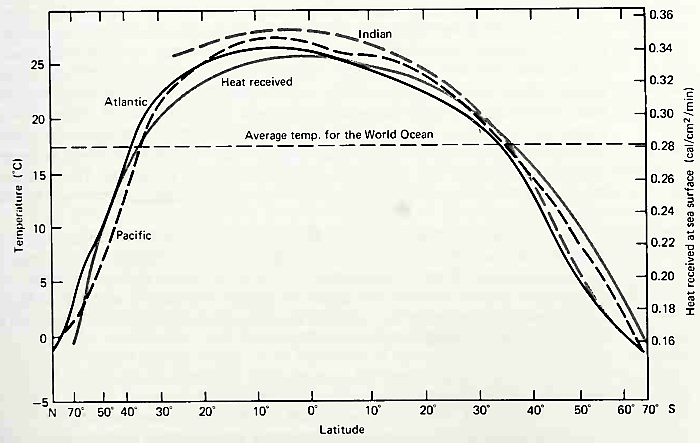
Figure 6-9. Mean zonal distributions of surface temperature for the three ocean basins and heat gain through the sea surface as a function of latitude. (Data from Sverdrup et al., Prentice-Hall, Inc.)
Blue green light is absorbed least. It does not cause much thermal excitation of molecules, but it is used for plant photosynthesis. It penetrates to about 100 m in the open ocean and to about 10 m in harbors and other nearshore areas.
Fig. 6-10 shows how radiant energy of several frequencies is absorbed at a particular location in the world ocean. Below about 100 m, there is virtually no penetration of light, because near-total absorption of radiant energy occurs at this depth. The zone between the surface and the depth of total absorption is called the photic, or lighted, zone.
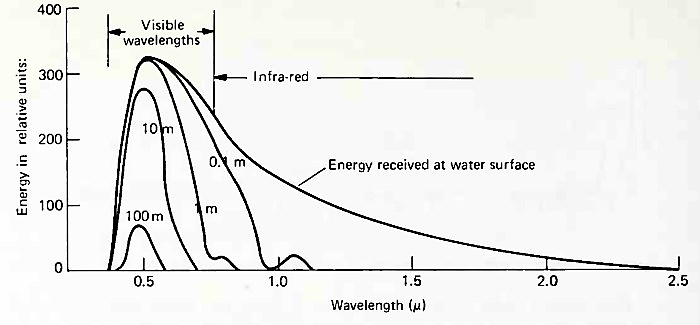
Figure 6-10. Energy spectrum of solar radiation at the water surface and at various depths. (After Sverdrup et al., by permission of Prentice-Hall, Inc.)
The blue green color of the ocean is caused by the minimum absorption of blue green light. Blue green light can be transmitted into, scattered, and transmitted out of water without being absorbed.
Sometimes, however, the color of the ocean is not blue green. For example, dissolved organic matter of yellowish brown color gives a green or greenish brown color to the ocean. There are red tides, caused by dinoflagellates (very small marine organisms) that impart a reddish tint to the ocean. In certain locations, such as off the coast of Norway, microorganisms called coccolithophore sometimes concentrate and impart a milky white color to the ocean. Suspended sediment may cause a muddy coloration near rivers and along shores where wave action is strong. As these sediments are carried to sea and sink, they form layers of suspended sediment called nepheloid layers.
These layers are composed of extremely fine particles held in a temporary, stable (nonsettling) suspension. They scatter light much better than does seawater and are detected by this difference at great depths in the ocean. Determining their distribution provicies information on sedimentation processes in the deep ocean.
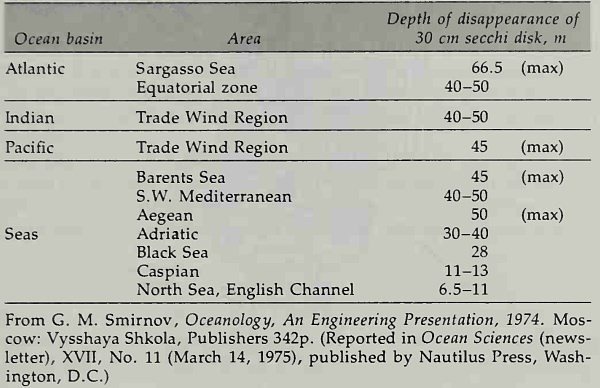
Table 6-2. Transparency of Seawater in the World Ocean
The transparency of seawater is important to plant life in the ocean and it is a subject of concern to biological oceanographers. It is measured in terms of the transmission of light introduced artificially in water at depths below the photic zone and in terms of the depth of disappearance of a white disk 30 cm in diameter viewed from the surface. The transparency of water in the world ocean is quite variable, as is seen in Table 6-2.
Date added: 2024-04-08; views: 590;
