Adenosine Triphosphate (ATP). Energy Transduction
DIFFERENT FORMS OF ENERGY are interconverted in biological systems. For this purpose, the cell uses phosphate compounds as the common currency of energy exchange. Adenosine triphosphate (ATP) is an energy-rich phosphate compound that is used as a special carrier of energy in the living cell. As shown in Fig. 1, ATP consists of an aromatic base called adenine, a five-carbon sugar called ribose, and three phosphate groups (α, ß, and γ-, with the α-phosphate linked to the ribose by an ester bond and the ß- and γ-phosphates linked together by two phosphoanhydride bonds.
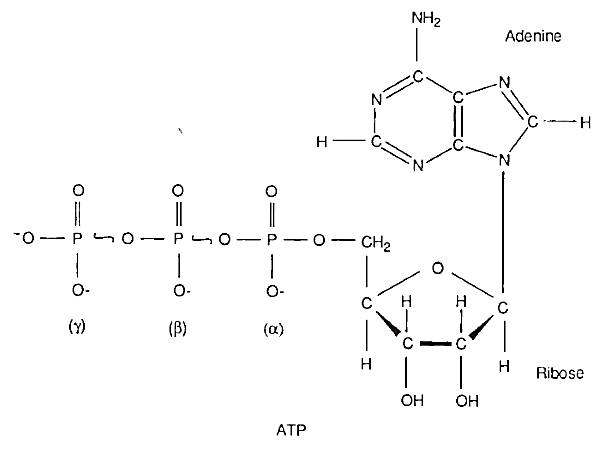
Figure 1
Despite the complex structure of the ATP molecule, only the ß- and y-phosphates of ATP are used for the transfer of energy in the cell. This part of the molecule is similar to pyrophosphate (PPi). In aqueous solutions, energy is liberated when the phosphoanhydride bonds of either ATP or PPi are cleaved. The amount liberated varies depending on the pH, temperature, and concentrations of divalent cations in the media, and, in some experimental conditions, the energy of ATP hydrolysis is higher than that of PPi (Table I).
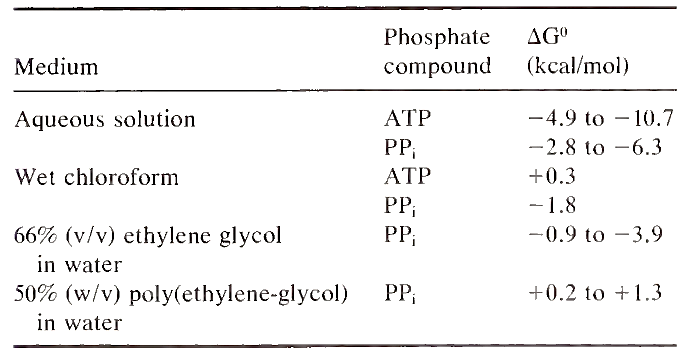
TABLE I. Energy of Hydrolysis of Phosphate Compounds in Media with Different Water Activities
We do not know why a complex molecule such as ATP is necessary as an energy carrier instead of a much simpler molecule such as PP; nor do we know how the presence of adenine and ribose in the molecule may alter the energy of hydrolysis of the phosphoanhydride bond of ATP. In the cell, ATP is hydrolyzed to either adenosine diphosphate (ADP) and orthophosphate (Pi) or to adenosine monophosphate (AMP) and PPi. The total concentration of ATP plus ADP and AMP in the cell varies between 2 and 10 mM. The proportion of the three species vary considerably with the energy status of the cell. The turnover of ATP in the cell is very high. In most cells, an ATP molecule is hydrolyzed a few seconds after its formation.
Energy Transduction. The hydrolysis of ATP is catalyzed by different enzymes that are capable of energy transduction. These enzymes use the chemical energy derived from the cleavage of ATP to perform mechanical work, to build up ionic gradients across membranes, or to promote the synthesis of new molecules. In most enzymes, ATP is used as a complex with Mg2+.
Until recently, the energy of hydrolysis of ATP and other energy-rich phosphate compounds were thought to be the same regardless of whether they were in solution in the cytosol or bound to the enzyme surface, and thus energy would be released and become available to the enzyme only after the phosphate compound had been hydrolyzed.
The sequence of events in the process of energy transduction was as follows:
(1) The enzyme binds the phosphate compound.
(2) The phosphate compound is hydrolyzed and energy is released.
(3) The energy derived from the cleavage of the phosphate bond is absorbed by the enzyme.
(4) The enzyme uses the energy absorbed to perform work.
(5) Products dissociate from the enzyme. With this view, energy was not required for binding reactants, nor for release of products from the enzyme surface.
More recent data have led to a different view, after it was discovered that the energy of hydrolysis of ATP and of other phosphate compounds varies greatly depending on whether they are in solution or on the enzyme surface (Table II). Studies of the catalytic cycle of different enzymes indicate that energy becomes available for the enzyme to perform work before the cleavage of the phosphate compound.
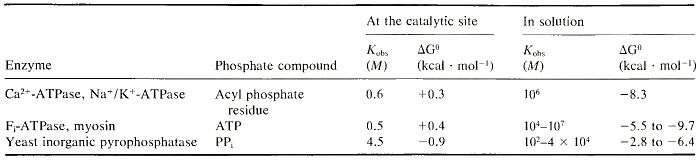
TABLE II. Variability of Energy of Hydrolysis of Phosphate Compounds
With this new view, the sequence of events for the hydrolysis of a phosphate compounds is as follows:
(1) The enzyme binds the phosphate compound.
(2) The enzyme performs work without the phosphate compound being hydrolyzed. This is accompanied by a decrease in the energy level of the phosphate compound; the presence of the phosphate compound allows the enzyme to assume a new conformation and in the transition, work can be performed.
(3) The phosphate compound is hydrolyzed in a process that energy change.
(4) The products of hydrolysis dissociate from the enzyme. In the reverse process, phosphate compounds such as ATP or an acyl phosphate residue are synthesized on the enzyme surface without the need of energy. Energy is then needed for the conversion of the phosphate compounds from “low energy” into “high energy.” The conversion is coupled with a conformational change of the enzyme. In this crucial step, different forms of energy are interconverted.
The changes in the binding environment at the catalytic site that are responsible for the change in energy level of a phosphate compound are not clearly understood at present. Experimental evidence suggests that one important factor is a change in the way that water molecules are organized at the catalytic site of the enzyme. In aqueous mixtures of different organic solvents, the energy of hydrolysis of ATP and PP; is similar to that measured on the surface of enzymes and is significantly smaller than that measured in totally aqueous solutions (Tables I and II). These findings can be interpreted according to the solvation energy concept discussed below.
Date added: 2023-05-09; views: 908;
