Processes Regulating the Composition of Seawater
Several processes regulate the chemical composition of the world ocean. They are of four general types:
1. Physical regulatory processes, such as temperature effects, evaporation, and freezing
2. Biological regulatory processes
3. Chemical regulatory processes, such as solubility, adsorption of ions on solid surfaces,- and involvement of ions in reactions between minerals and seawater
4. Geological and climatological processes.
The concentration of each chemical component dissolved in seawater is regulated by at least one, and often by several, of the above processes. When we compare the solubilities of all the elements dissolved in the ocean to the concentrations that exist, we find that just a few are present in saturation amounts. Of the major cations in the ocean, only calcium is near saturation; saturation is maintained by the dissolution or precipitation of solid calcium carbonate. Nitrogen and the inert, or noble, gases are near saturation; their concentrations are strongly regulated by temperature.
The amount of major anions in the sea is regulated by the formation of salt (NaCl) and gypsum (CaSO4) deposits in hot shallow embayments in arid regions or by the semipermanent removal of water to the continents during glaciation. The regulation of the major anions is mostly geological and climatological in nature: earth movements produce shallow embayments that desiccate and evaporate seawater, and changes in climate over geologic time regulate the amount of the ocean's water trapped on the continents as ice.
Adsorption, an important chemical process in the ocean, occurs when ions or molecules become attached to atoms at the surface of solids by weak electrostatic bonds. The strength of the attachment varies according to the composition of the solid and to the size and charge of the atoms involved. Small multicharged ions are adsorbed more strongly than are larger ions or ions having fewer charges.
Ions absorb more strongly than do molecules, particularly those that have a uniform distribution of positive and negative charges (nonpolarised molecules). As a consequence, ions and molecules exhibit an order of preference of adsorption on a surface, and strongly adsorpable ions or molecules can displace more weakly adsorpable ones from a surface. The process is called ion exchange.
The concentrations of sodium, potassium, magnesium, rubidium, cesium, and, to some extent, calcium are controlled by cation exchange on clay minerals brought to the oceans by rivers. In river water and in the ocean, the order of preference of adsorption on clays is magnesium, potassium, and then sodium.
The removal of these elements from seawater solution should be in that order, and the concentrations of these elements in the ocean should increase in the reverse order. However, potassium is most readily extracted from seawater by clay minerals because clay minerals (especially illite) incorporate potassium in their crystal structures. Consequently, the potassium ion is less concentrated in seawater than magnesium.
Magnesium concentrations in the interstitial water of marine sediments diminish with depth below the sediment-water interface more quickly than do the concentrations of potassium. Apparently, magnesium and, to a lesser extent, potassium are removed by adsorption on clays even after their burial in sediments.
The minor elements manganese, nickel, cobalt, zinc, and copper are greatly undersaturated because they are adsorbed on ferromanganese minerals in nodules on the ocean floor. In addition, apatite (the calcium phosphate mineral in fish-bone debris) adsorbs thorium, barium, strontium, and the rare earth elements, because, like the ferromanganese minerals, it presents active surfaces for adsorption. By these processes, trace elements are removed from seawater and concentrated in sediments and in nodules on the sea floor.
A subsidiary regulatory process is anion exchange. Little is known about anion exchange reactions in the ocean except that clay minerals can adsorb anions. Perhaps anion exchange accounts for the relative enrichment of bromine in sediments and to an extent is responsible for the uptake of phosphate in marine sediments.
Adsorption reactions are important in maintaining electroneutrality in seawater. The principle of electroneutrality applied to the world ocean means that the total of positive charges on dissolved cations must balance the total negative charges on the dissolved anions. Rivers introduce a surplus of positive charges to the sea in the form of the major cations Na+, K+, Ca2+, and Mg2+ produced by weathering of rocks on the continents. Adsorption removes the surplus from solution and maintains electroneutrality.
Chemical processes other than adsorption regulate the concentrations of the major ions in the ocean. For example, sodium and magnesium ions are incorporated in the minerals formed by the hydrothermal alteration of basalt rocks exposed at the sea floor. The removal of these ions disturbs electroneutrality and this imbalance is countered by adsorption reactions. Other reactions, such as precipitation, form crystalline minerals. Even though the formation of these crystals changes the concentration of major ions in seawater, electroneutrality persists because the crystals are electrically neutral.
Biological activity regulates the concentration of those elements involved in life processes. These are shown in Table 4-4. The elements regulated most by biological activity are oxygen, carbon dioxide, phosphate, nitrate, nitrite, ammonia, silicate, and calcium. Possibly vitamins and trace metals dissolved in seawater are regulated more than it seems inasmuch as these substances are all involved in the metabolism of organisms. Calcium is used by organisms to form carbonate shells; its concentration in the sea is regulated by the influence of biological activity on the carbonate equilibrium of the sea (to be discussed presently).
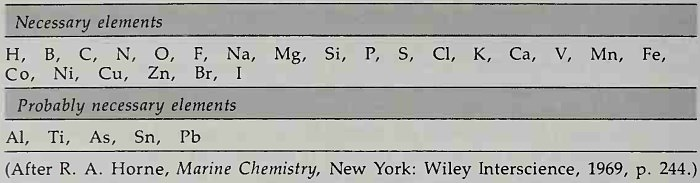
Table 4-4. Chemical Elements in Life Processes
The molecular ratios of several of the biologically important elements in water of the deep ocean are the following:

That is, for every molecule of phosphate there are 16 molecules of nitrate, and so on. When deep water wells up into the photic zone, plants extract materials to form their tissue and shells. They require the materials in the ratios:

When plants are grazed by animals, the elemental uptake is in the same proportions. Plants will extract all the available nitrate and phsophate and proportional amounts of the other elements, leaving the surface water with the ratios:

Eventually, the plant and animal materials fall to the sea floor as particles of dead tissue, shells, or excrement. Most of this material is attacked by bacteria and becomes redissolved in deep water. A small fraction becomes incorporated in bottom sediments where bacterial action continues, eventually consuming all the oxygen available (Table 4-5).
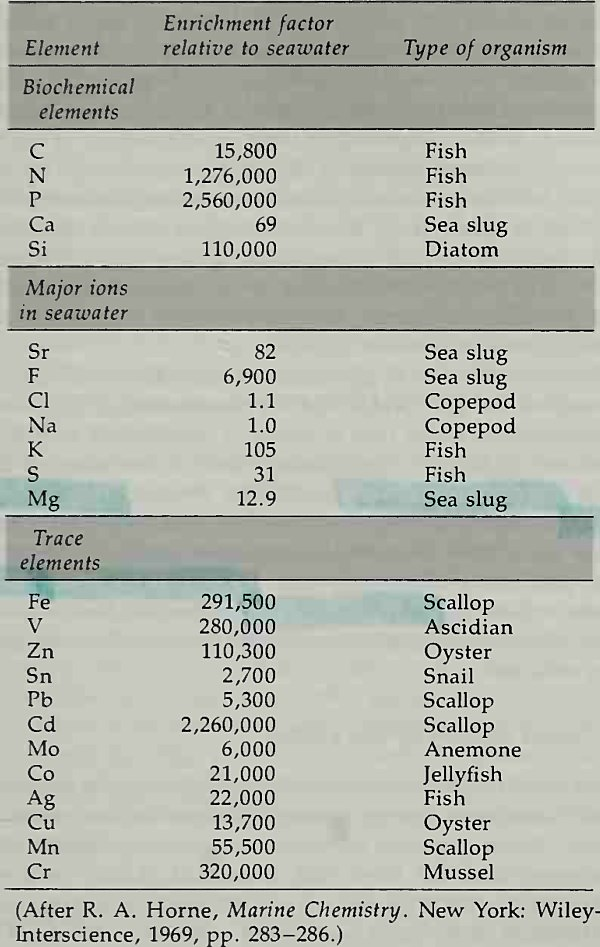
Table 4-5. Enrichment of Chemical Elements by Marine Organisms
Bacterial action is continued by sulfate-reducing bacteria that regulate the amount of sulfate and sulfide in the sediments and in other oxygen-deficient parts of the ocean. Because they break down organic debris, bacteria are instrumental in regulating the amount and composition of substances involved in biochemical processes in the ocean.
The organic particles formed from excreta and from the disintegration of oceanic organisms present adsorption surfaces that allow them to exert a regulating influence on the concentrations of trace elements and other cations.
Proteinaceous decay products, for example, associate with zinc, tin, lead, titanium, copper, silver, magnesium, aluminum, chromium, and nickel. Some organisms have the ability to concentrate trace elements by factors as high as 1 million, so significant amounts of these elements may exist in live organisms as well. Table 4-5 presents a list of enrichment factors for the biochemical elements, for some major ions in seawater, and for trace elements.
Physical processes in the ocean tend to regulate the amount of water in seawater rather than the amount of dissolved substances. The formation and melting of ice, evaporation, and precipitation of rain or snow change the absolute concentrations of dissolved substances by dilution or removal of water, but the amounts of dissolved materials remain in the same proportions.
Mixing of water from the surface layer of the ocean with deeper water can influence the vertical distribution of elements involved in biochemical processes. The lateral distribution of biochemical elements is regulated by horizontal water movement, such as the major circulation in oceanic gyres and the mixing motion of water masses.
The net behavior of all processes controlling the concentration of an element dissolved in the ocean can be summarized by comparing the element's concentration to the rate at which it is added or removed from the ocean. The quotient of these is the residence time of the element in the ocean.
Elements with short residence times (Al, Ti, Fe, Cr) are reactive and form insoluble solids before reaching the ocean or soon after arriving in the ocean. In the latter case, they form ferromanganese nodules and minerals, such as zeolite and glauconite. Elements with long residence times (Na, K, Ca, Mg) are characterized by high solubility. Divalent cations have shorter residence times than their monovalent counterparts because they are adsorbed more easily by clay minerals.
Date added: 2024-04-08; views: 636;
