Formation of Residues in Chemical Processes
Chemical reactions do not lead exclusively to the desired product but also to residues which may pollute the environment. To understand the reasons for the formation of residues in chemical production processes, and to understand the possibilities for modifying these processes, the general conditions for chemical reactions must be considered.
As an example an equilibrium reaction is considered in which a starting material A reacts with a reaction partner В to give a product P:
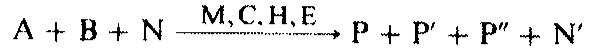
where A is the starting material, В the reaction partner, N a secondary constituent of A and B, N' the reacted secondary constituent, M the reaction medium, C a catalyst, H an auxiliary, P' a joint product, E the energy, P the product, and P" a side product or byproduct.
The term “residues” denotes all components that take part in the reaction and do not give the desired target product P.
Incomplete Conversion. In equilibrium reactions, components A and В do not react with each other to completion. One of the two substances A or В is generally used in excess to increase the extent of conversion to the other at equilibrium. Occasionally, the component present in excess is the reaction medium for the chemical conversion. The excess amount of A or В remaining at the end of the reaction can contain a higher or lower content of impurities, and must be disposed of in an environmentally friendly manner if it cannot be recycled directly after physical or chemical treatment.
Associated Substances or Impurities in Raw Materials. The appearance of byproducts or residues in chemical reactions can also be due to associated substances or impurities present in the starting materials. This applies especially to the winning of inorganic and organic basic materials from mineral, fossil, or biological raw materials. Examples of associated substances include the following:
1) Hydrogen sulfide in the processing of sour natural gases and mineral oils
2) Sulfur dioxide and dust emissions in the production of acetylene and synthesis gas by the formerly widely used coal-based processes
3) Contamination of wastewater by dissolved decomposition products of lignin (lignosulfonic acids, etc.) in the production of pulp from wood or other vegetable raw materials.
Joint Products. Another characteristic of many syntheses is the formation of joint products P', the quantity of which is coupled to the quantity of the target product P.
In inorganic syntheses, for example, the type of joint product can depend on the form of the raw material component A in the available mineral or ore. For example, when sulfidic nonferrous ores are treated in pyrometallurgical processes, S02 is formed as the joint product, and the joint product FeS04 is formed during the digestion of ilmenite (FeTi03) in the production of the white pigment Ti02 by the sulfate process. In the production of phosphoric acid from phosphate rock by the sulfuric acid process the calcium and fluorine content of apatite even leads to two joint products, namely, gypsum and hydrogen fluoride.
In organic syntheses, starting material A often contains a reactive group that is split off in the form of a joint product during the reaction. In many cases, halogens or sulfonic acid groups are eliminated, forming acids or salts, and are replaced by other substituents. Also, selective reduction of organic compounds in aqueous solution by metals such as iron or zinc leads to formation of the corresponding metal salts or oxides as joint products.
By-products or Products of Secondary Reactions. By-products PN also reduce the yield of product and must be removed during the course of reaction or during processing of the reaction mixture. They can be formed by continuance of the reaction; e.g., in chlorination processes intended to produce monochlorinated compounds, higher chlorinated products can also be formed.
Reaction Medium. In chemical reactions and separation processes in the liquid phase (e.g., organic solvents or water), solutions or multiphase systems with at least one liquid component are present. The reaction medium or process medium M in these processes is either a solvent, and therefore not a contributor to the reaction, or both a solvent and a reaction partner, and is sometimes a catalyst.
Concentrated mineral acids (e.g., sulfuric acid or oleum) are often used in excess for digestion of inorganic raw materials or for production of reactive intermediates from relatively unreactive organic starting products. That proportion of acid not required for product formation must be either regenerated or disposed of in an environmentally friendly manner. Both routes are costly. Reprocessing is particularly difficult if the acid must be diluted or partially neutralized, or if salts have to be added to the solution in order to recover the product.
When water is used as the reaction medium, wastewater is produced that is sometimes highly contaminated with organic or inorganic substances.
Catalysts can also contribute to the formation of residues. Heterogeneous catalysts must be replaced when their activity is exhausted. In the case of homogeneous catalysts, either the catalyst component is removed along with the used process medium, or an additional processing stage is required to separate the used catalyst from the product.
Auxiliaries can be necessary in some reactions. Like catalysts, these take part in the process without being used up in the chemical reaction. For example, multiphase reactions sometimes require the use of surface-active agents to increase the interfacial surface area or accelerate the transfer of matter. These substances appear as residues or in wastewater when the reaction mixture is processed.
Process Energy. The process energy (electricity, heat) required must also be included in the environmental assessment of chemical processes. Generation of this energy from primary energy carriers also leads to emissions.
Date added: 2023-09-23; views: 601;
