Recovery and Utilization of Residues in the Production of Viscose Staple Fiber
General Features of Viscose Staple Fiber Production [89]. Cellulose from wood is the most important starting material for staple fiber production by the viscose process. Cellulose xanthate is obtained by converting cellulose to sodium cellulose followed by treatment with carbon disulfide.
Reaction scheme
1) Production of alkali cellulose Cellulose + NaOH → Alkali cellulose
2) Aging
3) Viscose formation (dissolution of alkali cellulose by addition of carbon disulfide)
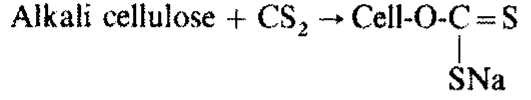
Side reaction:
2 CS2 + 6 NaOH → Na2CS3 + Na2CO3 + 3 H2O + Na2S
4) Ripening
5) Regeneration of cellulose fibers in the H2S04-Na2S04-ZnS04 coagulation bath
Cell-0-CS2Na + NaHS04 → Na2S04+ CS2 + Regenerated cellulose
Side reactions:
Na2CS3+H2S04 → Na2S04 + H2S + CS2
Na2S + H2S04 → Na2S04 + H2S
Na2C03+H2S04 → Na2S04 + H20 + C02
Cellulose xanthate is dissolved in aqueous sodium hydroxide solution to give a viscous liquid—the viscose. The sodium hydroxide solution, which is added in excess, also results in the formation of by-products such as sodium trithiocarbonate and sodium sulfide. In addition sodium carbonate is formed.
The ripening of viscose is stopped after a certain time, depending on the nature of the required end product, and the cellulose is precipitated. This is performed by injecting the filtered spinning solution through fine nozzles (the orifices of the spinneret) into an acid bath. The main reaction occurring in the acid bath is the coagulation of viscose, and CS2 is formed. In side reactions, salts formed in the preparation of the spinning bath decompose to H2S, C02, and Na2S04 (step 5).
As the strand or slubbing is led out of the spinning bath, subsequent degassing occurs, in which carbon disulfide and hydrogen sulfide dissolved in the acid spinning bath partly evaporate. The spun fibers then pass through several aftertreatment baths. In the course of this, adhering sulfuric acid is washed off. A subsequent degassing of carbon disulfide and hydrogen sulfide also occurs in the wash baths, which are mostly hot.
For production of 1 t of staple fiber, up to 350 kg of CS2 is required. About 25 % of the CS2 decomposes to H2S and C02. The schematic of the entire process is shown in Figure 14.
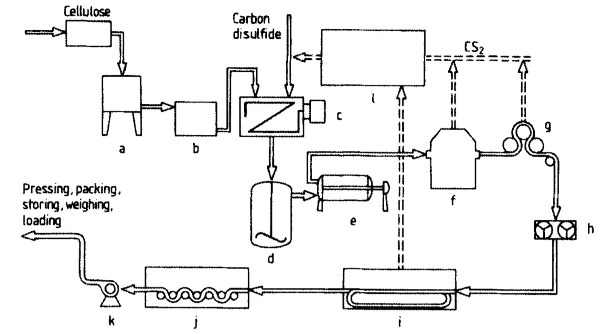
Figure 14. Schematic of viscose staple fiber production: a) Mashing; b) Ripening; c) Sulfidization; d) Dissolving; e) Filtration; f) Spinning; g) Stretching; h) Cutting; i) Washing and aftertreatment; j) Drying; k) Transport; l) CS2 recovery
The Problem. During the production of staple fibers by the viscose process, toxic waste gases containing carbon disulfide and hydrogen sulfide are formed. In the conventional process, those waste-gas streams that contain mainly carbon disulfide were collected in a waste-gas system with associated recovery.
These waste gases arise during deaeration and exhaustion in viscose production and during aftertreatment of the viscose staple tow. In the recovery plant, after the separation of the hydrogen sulfide fraction, carbon disulfide was adsorbed on activated carbon. Up to about 65 % of the carbon disulfide used was recovered.
The greater part of the other sulfur-containing waste gases—ca. 60-90 kg of sulfur in the form of CS2 and H2S per tonne of staple fiber—previously entered the waste air. This part originated mainly from the spinning process and also from the spinning bath components discharged with the fibers, from which the gases are released by diffusion in the later treatment stages. In the original procedure, the waste-gas sources in the process had to be exhausted with very large quantities of air to keep waste-gas concentrations safely below the in-plant threshold limit.
The specific quantity of waste gas was therefore extraordinarily high (ca. 235 000 m3/h at an output of 55 000 t of viscose staple fiber per year). The extracted gases, greatly diluted, reached the open air via a tall stack. As a result, nuisance odors often occurred in the surroundings of viscose staple fiber plants because the threshold odor concentration of hydrogen sulfide, at ca. 0.005 mg/m3, is extraordinarily low. Disposal of the waste gas failed because of its large volume and low concentration.
Considerable quantities of sodium sulfate and zinc sulfate are also formed during regeneration of cellulose fibers. Part of the sodium and zinc sulfates is discharged from the spinning bath together with the viscose fibers and enters the wastewater through the aftertreatment of the fibers.
Solution [89] - [92]. To solve the waste-gas problem, a recycling scheme was developed by Hoechst and Süd-Chemie, Kelheim/Germany, and realized in 1975 -1979. In this process, 80% of the viscose waste gases were collected. In the following years, further increase of collection to the current value of 90 % was achieved, and the waste- gas volume was simultaneously reduced considerably. In the recycling scheme, sulfur- containing waste gases from viscose staple fiber production (Hoechst) are supplied to a neighboring sulfuric acid plant (Süd-Chemie) where they are used as a raw material or as combustion air.
The sulfur compounds contained in the waste gases are combusted to S02, C02, and H20, and the S02 is processed to H2S04. The sulfuric acid produced can be used again directly for the coagulation bath. The new process concept required substantial changes and new developments in both plants. In addition it provided the preconditions for improved recovery of carbon disulfide in the viscose plant.
New Concept of Waste-Gas Capture during Viscose Staple Fiber Production. As a result of the basic chemical reactions in a sulfuric acid plant and the desired conversion of nearly 100 %, the ratio of the quantity of combustion air to sulfur is fixed. If the entire amount of waste gas formed in a conventional viscose staple fiber plant were to be used as combustion air in a sulfuric acid plant, about 30 times as much sulfuric acid would have to be produced as the staple fiber plant consumes.
The quantity of lean waste gas from the viscose plant must therefore first be matched to the much lower combustion air requirement of the sulfuric acid plant. This can be achieved by appropriate reduction of the air exhaustion in the viscose staple fiber plant. However, this is possible only if, at the same time, risks to safety as a result of exceeding the in-plant threshold limit value and of the occurrence of explosive gas - air mixtures are avoided.
For this purpose, waste gases from the various process stages are assembled in two separate systems: waste-gas system 1 (“rich gas”) is used for the collection of combustible but not explosive gases (waste-gas concentration > explosive range), and waste-gas system 2 (“lean gas”) is used for the collection of waste gases of low concentration (waste-gas concentration < explosive range). Extensive changes to the viscose staple fiber plant were necessary to minimize waste-gas emission:
1) The quantity of carbon disulfide required in viscose production was reduced by developing a special aging process.
2) Because of the new concept of collection of waste air from viscose production, the spinning machines, stretching and tow washing, and subsequent waste-air treatment, today most of the carbon disulfide can be recovered from the waste gas.
3) To recover hydrogen sulfide from the spent spinning bath acids as completely and at as high a concentration as possible, a vacuum degassing plant was constructed. The concentrated waste-gas stream of ca. 200 m3/h can be utilized directly in the sulfuric acid plant (rich gas).
4) By careful enclosure of the spinning machines and the apparatus in the acid station and by using novel exhaustion systems, the total waste-air stream of 180 000 m3/h (STP) has been reduced to 10 000 m3/h (STP). The entire spinning machine waste air can therefore be routed via the waste-gas purification plant (CS2, H2S). Lean gas from the acid station is used as combustion air in the sulfuric acid plant.
5) Finally, an extensive waste-air collection and piping system had to be constructed. Corrosion problems in the lines had to be ruled out by selecting suitable materials.
6) To ensure safety, a series of additional measures was required: e.g., a largely flangeless construction and reduced-pressure mode of operation of the waste-gas system to avoid leaks; installation of a process measuring and control technology with emphasis on safety; and construction of an additional exhaustion system on the spinning machines for manual operating procedures.
Hydrogen Sulfide Recovery. Waste air from the production installations (spinning, stretching, tow washing), which is contaminated with CS2 and H2S, is freed from H2S by a multistage wash with sodium hydroxide solution (Fig. 15, a). In the course of this, an NaSH-containing spent lye is formed. Subsequently, CS2 contained in the waste gas is adsorbed on activated carbon (b) and then desorbed with steam (Supersorbon process).
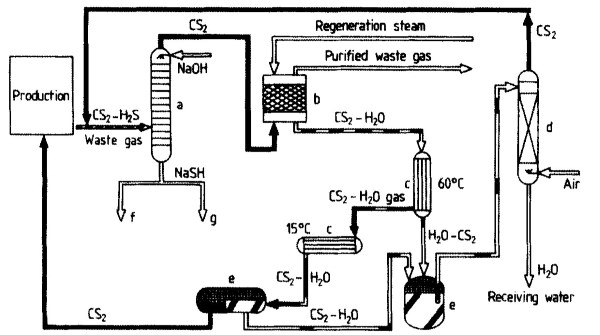
Figure 15.Carbon disulfide recovery in viscose staple fiber production: a) Wash column; b) Adsorption on activated carbon; c) Condenser; d) CS2 stripper; e) Separator; f) Zinc precipitation; g) Acidification (H2S used for H2S04 production)
The desorbate water -CS2 vapor is precondensed at 60 °С (c). The gaseous residue is then condensed at 15 °С, and the condensate is drained off into the separator (e). The aqueous phase obtained, together with the precondensate, is stripped of CS2 in a column (d). The CS2 is returned to the production process.
Zinc Recovery. Part of the spent lye from the gas wash (a) is used to precipitate zinc (f) from the stretching bath and from the wastewater of the tow wash. By addition of H2S04 to the precipitated zinc sulfide, zinc sulfate is recovered for the spinning process. During zinc precipitation, a small portion of zinc polysulfide is formed that cannot be converted to zinc sulfate. This portion must be discharged.
Hydrogen Sulfide Utilization. The H2S content of the remaining spent lye (g) is released by adding H2S04 and utilized in the neighboring sulfuric acid plant together with H2S from zinc sulfate recovery and waste gas (CS2, H2S) from the spinning bath regeneration.
Sodium Sulfate Recovery. In the spinning bath regeneration, the spinning bath is partially evaporated. In the course of this, sodium sulfate is obtained in a marketable form by crystallization. The overall flow sheet of the new process is shown in Figure 16 A.
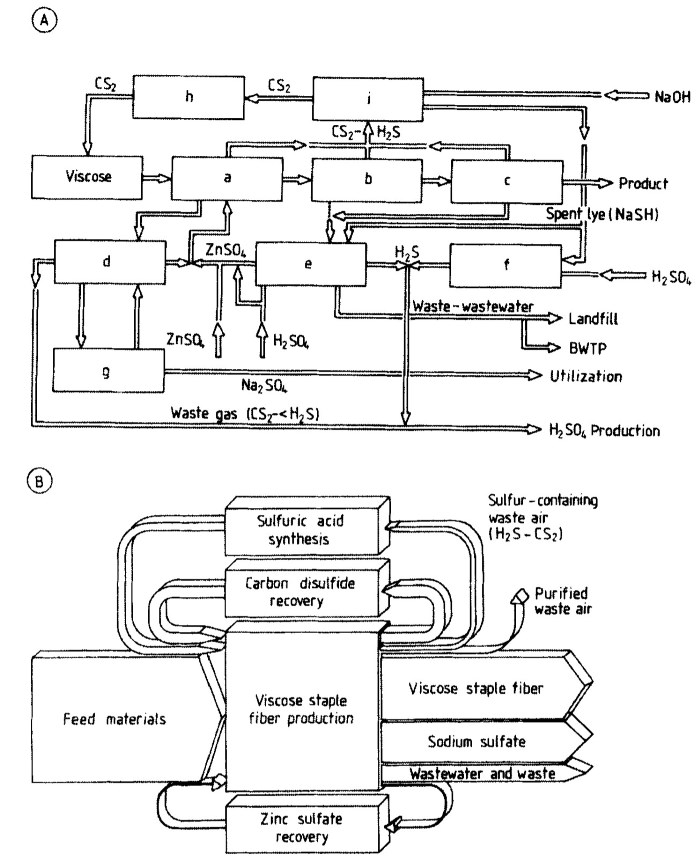
Figure 16. Residue utilization in viscose staple fiber production:
A) a) Spinning; b) Stretching; c) Tow wash; d) Spinning bath regeneration; e) Zn recovery; f) Spent lye degassing; g) Na,S04 crystallization; h) Recovery; i) WashingBWTP = Biological wastewater treatment plant
B) Overall balance of the integrated pollution control solution
Supplementary Engineering Equipment for Process Rearrangement. To be able to utilize the lean gas stream, which is saturated with water vapor, in the sulfuric acid plant, a new wet-dry catalytic process for sulfur combustion was developed jointly by Süd-Chemie and Lurgi. In this process the water vapor from the S03 - S02 gas mixture is removed after the third catalytic stage by condensation in a new absorption process. The dry S03 - S02 gas mixture then passes through the fourth catalytic stage. Depending on the degree of dilution of the waste gases (with N2 and C02 as inert constituents), the new process gives a sulfuric acid yield of 99.1-99.4%.
Pollution Control Balance. The overall balance of the integrated pollution control is shown in Figure 16 B. As a result of collecting the waste-air streams, ca. 90% of the original emissions of sulfur compounds from the plant can be avoided, corresponding to ca. 5000 t/a (calculated as S). Only the waste air arising during manual operations on the spinning machines, which is greatly diluted as a result of the additional exhaustion, must be discharged from the stack as before. Apart from rare plant upsets, the nuisance odor in the surroundings of the plant is a thing of the past.
Moreover, during spinning bath regeneration, about 40 000 t/a of anhydrous sodium sulfate is recovered. This corresponds to about one-half of the amount formed in the overall viscose process (including neutralization steps). The remaining wastewater enters a Biohochreactor for degradation of the organic materials (dissolved cellulose ingredients).
The solution found here to the viscose waste-gas problem dispenses with additional energy requirement in the form of electric power, fuel gas, or natural gas. No new waste materials are produced. The operating costs of the process depend basically on the market price of sulfuric acid. The process scheme has by now been applied internationally in several viscose plants.
Date added: 2023-09-23; views: 628;
