Integrated and Additive Concepts of Environmental Protection
The environmental demands placed on the production process can be met with the aid of integrated or additive (end-of-the-pipe [34]) concepts.
The sustainable development model demands a new orientation of environmental politics. Therefore innovations in all areas of industrial environmental protection are the most effective tools. These will lead to an efficiency revolution.
Its elements can be characterized as follows:
- Process innovation: Production of the same or similar products using less raw material and energy and giving lower pollutant output as waste gas, waste and polluted wastewater, i.e., development of environmentally friendly processes.
- Material cycles: recycling of residues and product wastes can reduce use of resources.
There the adopted way of integrated environmental protection must be consistently followed further.
Production-Integrated Environmental Protection. According to the definition of the VCT, integrated environmental protection is production-linked, and that definition is used in this article. Thus, production-integrated environmental protection means measures taken to reduce, prevent, and utilize residues.
The reduction and prevention of residues can be achieved by
1) Improving the chemical process with the aid of new synthesis routes. For example, in the production of aromatic amines, chemical reduction with iron chips is replaced by catalytic reduction by hydrogen.
2) Shifting the equilibrium. The use of more favorable reaction conditions can cause the position of the equilibrium to be shifted so that one of the two components A or В is almost 100% reacted. This can be achieved by using the second component in excess, by removing the product, or by using more favorable temperature or pressure.
3) Improving selectivity. A very effective method of reducing the amounts of residues and improving the yield is to increase the selectivity of the chemical reaction. Examples of this include the following:
- Improvement of the selectivity of catalysts, e.g., by using catalysts that lower the rate of an undesired side reaction
- Maintenance of high catalytic activity, e.g., by avoiding contact poisons or by developing simple reaction methods
- Optimization of reaction conditions, e.g., by utilizing differences in the reaction kinetics of the main reaction and the side reaction, more favorable temperature profiles and residence times, or more suitable reactors
- Recycling of the side product (if the side reaction is reversible)
4) Developing new catalysts, e.g., in production of polypropylene without generation of wastewater using improved metal - organic catalysts.
5) Process optimization.
6) Changing the reaction medium. If water is replaced by an organic solvent in syntheses, contamination of wastewater can often be drastically reduced. However, environmentally friendly solvent handling involves not only recovery of the solvent from liquid media but also prevention of losses to the atmosphere during storage, transport, production, and subsequent processing. This can be achieved, for example, by adsorptive recovery of solvents from the gas stream.
7) Using raw materials of higher purity.
8) Replacing or eliminating auxiliaries that have a harmful effect on the environment (e.g., chlorinated hydrocarbons).
Gaseous, liquid, or solid residues whose formation during the production process cannot be avoided, even under optimum operation conditions, can often be reused (see. Fig. 5) by methods such as
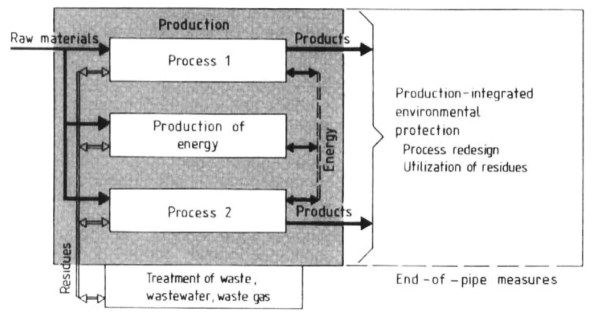
Figure 5. Approaches to environmental protection in chemical production
1) Internal utilization, e.g., of the auxiliaries employed in the process, by processing and recycling directly into the process. In the simplest case, the substance can be used directly after separation from the products or process streams (recovery of volatile components of solvents). In other cases, a physical or chemical processing stage is necessary to remove impurities from the recycled components or convert them into a reusable form.
Examples include
- Recovery of sulfuric acid from waste sulfuric acid by concentration or decomposition and reprocessing
- Recovery of organic solvents from solvent residues, solvent - product mixtures, aqueous solutions, and production residues
- Thermal decomposition of residues of chlorination processes to give pure hydrochloric acid
2) external utilization of the residue, i.e., as a raw material for the manufacture of other products in a separate production plant.
Reutilization of residues by linking of production processes is not another form of residue disposal, but enables resources to be used as economically as possible. However, this does lead to additional interdependencies, which may decrease flexibility.
Additive Environmental Protection. If the technical and economic possibilities for preventing, avoiding, or reusing residues (as part of a production-integrated environmental protection program) are exhausted, additive environmental technologies must be used:
1) Disposal of waste by land-filling and incineration
2) Techniques for purifying waste gas and wastewater
Of course, not all problems of environmental protection in the chemical industry can be solved by new process concepts based on the principles of prevention and utilization of residues. In many cases, additive processes are still necessary.
In the past, the main emphasis was on additive environmental protection employing conventional purification and disposal methods. Nevertheless, methods of recovery and utilization have long been used in the chemical industry, and can even be found at the beginning of industrial production.
In the chemical industry a long tradition exists of reprocessing reaction mixtures (e.g., by solvent extraction or distillation), with recovering of auxiliaries (e.g., organic solvents), and recycling these into the production process (internal recycling). For example, a desired product that is dissolved in a solvent following an extraction process can be recovered only by distilling off the extraction agent.
This yields the solvent in a pure form that can be recycled, and such recycling is an inherent part of the production process. It is also a “prevention” since the residues remain in the production plant [39]. Changes in the production process aimed at increasing the yield or reducing the generation of residues have always been tasks of chemical research. Nevertheless, the technology of integrated environmental protection is becoming increasingly important today because current requirements with respect to the prevention and utilization of residues are much more stringent than ever before.
Glauber, an early exponent of technical chemistry, described in 1648 the conversion of wine yeast. Yeast is a by-product of wine production and its conversion lead at that time to new products coupled with a partial wastewater recycle (Figure 6). Pressing the yeast cake yielded wine for vinegar production. Distillation of the residues yielded spirits and further processing gave potash and tartar. Potash was supplied to the dyestuffs industry and tartar sold to hatters.
The acid tartar-containing wastewaters were recycled in part. Some was used in treatment of copper ores to yield copper. Glauber also stressed the economic advantages of this process: Raw material costing 4 Thaler yielded an income of 16 Thaler from the products. This is an early example of an integrated production method in utilization of a by-product and reduction of polluted wastewater with added benefit.
Product-Integrated Environmental Protection. The concept of product-integrated environmental protection should be distinguished from that of production-integrated environmental protection. The former includes the utilization of product waste (e.g., the recycling of plastics) and the development of environmentally friendly products. These measures are also part of an overall environmental protection strategy of the chemical industry. They form a separate subject that is not discussed here.
Date added: 2023-09-23; views: 698;
