Reutitization Plant for Organohalogen Compounds
Introduction. Organic halogen compounds, especially those containing bromine and fluorine, are intermediates in the synthesis of pharmaceuticals, plant-protection agents, special high-performance plastics, and liquid crystals for optical displays. These intermediates must be produced in highly pure state. They are therefore separated from by-products formed during the chemical synthesis in later purification stages.
Since the by-products are often not directly reutilizable, they are disposed of in a waste incineration plant. Up to now only a recovery of the energy content is possible; a chemical reutilization of the by-products is not. Purification of the flue gas does not lead to reutilizable sustances.
The objective was not only to utilize the calorific values of these by-products or residues but also to reuse them chemically. This was achieved by thermal oxidation (incineration) with simultaneous neutralization to give potassium halides that are subsequently dissolved in water. The salt solution obtained is used for the recovery of potassium bromide, potassium fluoride, and potassium chloride as raw materials.
Method of Operation (Fig. 21). Liquid organic residues, which contain mainly bromine compounds with some chlorine and fluorine compounds, are fed through jets into a vertical cylindrical oxidation chamber (incinerator, a). The essential feature of the oxidation chamber is a special burner system that causes intensive turbulence of liquid and gaseous residues, fuel, and combustion air. An auxiliary flame produced by a special burner fired with natural gas is necessary to establish and ensure continuous operation of the oxidation process.
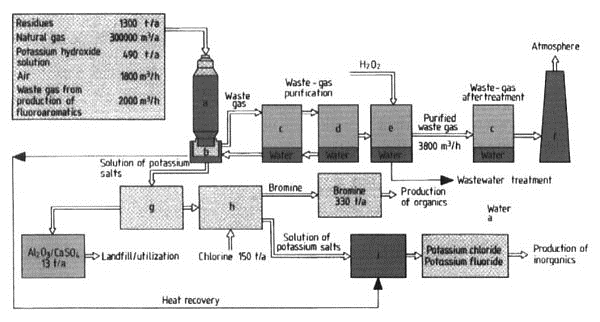
Figure 21. Recovery of bromine from residues containing brominated organics: a) Incineration and salt production (1200 °С); b) Quench cooling (85 °С); c) Scrubber (two stages); d) Electrostatic filter; e) Scrubber (one stage); f) Stack; g) Filtration; h) Bromine recovery; i) Evaporation
This burner produces a very intensely radiating flame that reduces the mixing zone within the oxidation chamber. Intensive mixing of the gases ensures complete reaction, which results in comparatively low emissions of NOx and CO. The high calorific value of the organohalogen residues ensures that the thermal energy necessary for the process is liberated.
Waste air from the production of halogenated aromatics is used to form part of the combustion air, so that the residues present in the waste air (e.g., fluorine compounds) can also be treated in the recovery process.
The residues are converted to hydrogen halides in the oxidation chamber at 1200 °С and a residence time of at least 2 s. The hydrogen halides are then immediately neutralized by the simultaneous spraying of potassium hydroxide solution into the chamber, to form potassium halides. This prevents the formation of free hydrogen halides or of elementary halogens according to the Deacon equation. In the case of bromine, the equation
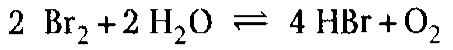
is shifted completely to the right by immediate neutralization of the hydrobromic acid. The equilibrium reaction is then no longer reversible, and complete conversion of the residues to hydrogen halides and, ultimately, potassium halides occurs.
This also avoids the secondary reaction
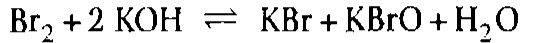
which occurs if the potassium hydroxide solution is added to the waste gas at a later stage, as in conventional exhaust-gas purification.
The potassium salts that are liquid at > 800 °С flow partially down the walls to the bottom of the oxidation chamber and are dissolved in the water bath in the quenching vessel (b) directly below. The vapor-phase fraction of the salt forms aerosols in the quenching vessel on cooling to ca. 85 °С. The concentrations of these aerosols are then reduced in the multistage waste-gas purification equipment (c-e) to levels below the limit values stipulated by Regulation 17 of the German Federal Antipollution Law (17. Verordnung Bundesimmissionsschutzgesetz 17. BImSchG). In this equipment, waste gas is passed successively through two jet scrubbers (c), a condensation electrostatic filter (d), and a packed column fed with H202 (e).
The liquid level in the quenching vessel is kept constant by adding liquor from the waste-gas scrubbers. Thereby some evaporation of the salt solution occurs.
Insoluble components of the salt liquor (ca. 1 % of the residues treated) are removed by filtration. These components consist mainly of insoluble inorganic salts and metal oxides; they can be dumped or, preferably, reutilized.
Elementary bromine is obtained from the filtered salt solution by reaction with chlorine in a further process step. Potassium chloride and potassium fluoride are recovered by evaporation of the remaining solution (with heat recovery from the quenching vessel) and are internally recycled.
The purified waste gas (see above) then undergoes a final purification by passing through a two-stage waste-gas scrubber in a production plant for inorganics (see Fig. 21).
Advantages of the Process, The formation of dioxins is prevented by the high oxidation temperature (1200 °С), the residence time of 2 s in the reactor, the mainly homogeneous temperature profile in the oxidation chamber, and the quench cooling of the reaction gases from 1200 to 85 °С. Measured concentrations are < 0.1 ng/m3 toxicity equivalents dioxins and furans.
Known alternative methods for treating liquid residues either have disadvantages or offer no advantages:
1) Hydrogenation process The high fluorine content of the residues leads to problems with construction materials. Elementary bromine is not recovered. Production of the hydrogen required for the process is energy intensive.
2) Plasma process Since the plasma burner can be used only as a pre-stage before the subsequent oxidation with air or pure oxygen, the consumption of electrical energy, and the cost of the plant are excessively high. Further recovery of the halogens will be similar to that used in the recommended process. The ecological balance of this process is therefore very unfavorable.
3) Pressure gasification A mixture of CO, C02, and hydrogen halides is formed at ca. 1600 °С. The hydrogen halides must be separated by waste-gas purification to recover the halogens. The formation of elementary bromine in the gasification process is problematic and would lead to considerable difficulties in waste-gas purification.
4) Chemical reverser In a converter tube clad with a special ceramic, a reductive atmosphere with a hydrogen excess at very high temperature is produced by combustion of propane under pressure. The fluid organohalogen compounds are decomposed in this temperature region.
For the substances mentioned above containing high levels of aromatics, the mixture would have to be led through a combustion chamber before entering the reactor to convert the molecules into suitable fragments that are able to react with hydrogen in the cracking process.
The later process steps, such as quenching and gas purification, would be very similar to those in the process initially described. It would be complicated to include the use of waste air as combustion air or the use of heat of reaction in the concept of the chemical reverser.
Date added: 2023-09-23; views: 625;
