Organic Compounds. Biology
The vitalist position remained strong, however. Even if it became necessary to concede that the law of conservation of energy held for living systems as well as non-living; or that both bonfires and living animals consumed oxygen and produced carbon dioxide in similar fashion, these represented merely over-all limitations—like saying that both human beings and mountain tops were composed of matter. There still remained the vast question of detail within that limitation.
Might it not be that living organisms, though composed of matter, were made up of forms of matter not quite like that of the inanimate world, for instance? This question almost seemed to answer itself, in the affirmative.
Those substances that abounded in the soil, sea, and air were solid, stable, unchanging. Water, if heated, boiled and became vapor, but could be cooled to liquid water again. Iron or salt might be melted, but could be frozen once more to the original. On the other hand, substances obtained from living organisms—sugar, paper, olive oil-seemed to share the delicacy and fragility of the life forms from which they were derived. If heated, they smoked, charred, or burst into flame, and the changes they under-went were irreversible; the smoke and ash of buming paper did not become paper again upon cooling. Surely, then, it might be fair to suppose that two distinct varieties of matter were being dealt with here.
The Swedish chemist, Jons Jakob Berzelius (1779-1848), suggested, in 1807, that substances obtained from living (or once-living) organisms be called "organic substances," while all others be referred to as "inorganic substances." He felt that while it was possible to convert organic substances to inorganic ones easily enough, the reverse was impossible except through the agency of life. To prepare organic substances from inorganic, some vital force present only in living tissue had to be involved.
This view, however, did not endure for long. In 1828, a German chemist, Friedrich Wohler (1800-82), was investigating cyanides and related compounds; compounds which were then accepted as inorganic. He was heating ammonium cyanate and found, to his amazement, that he obtained crystals that, on testing, proved to be urea. Urea was the chief solid constituent of mammalian urine and was definitely an organic compound.
Wohlefs discovery encouraged other chemists to tackle the problem of synthesizing organic substances out of inorganic ones, and success followed rapidly. With the work of the French chemist, Pierre Eugene Marcelin Berthelot (1827-1907), there remained no question that the supposed wall between inorganic and organic had broken down completely. In the 1850s, Berthelot synthesized a number of well-known organic compounds, such as methyl alcohol, ethyl alcohol, methane, benzene, and acetylene from compounds that were clearly inorganic.
With the development of appropriate analytical techniques in the first decades of the nineteenth century, chemists found that organic compounds were made up chiefly of carbon, hydrogen, oxygen, and nitrogen. Before long they learned to put these substances together in such a way that the resulting compound had the general properties of organic substances but did not actually occur in living creatures.
The latter half of the nineteenth century saw myriads of "synthetic organic compounds" formed, and it was no longer possible to define organic chemistry as being the study of compounds produced by life forms. To be sure, it was still convenient to divide chemistry into two parts, organic and inorganic, but these came to be defined as "the chemistry of carbon compounds" and "the chemistry of compounds not containing carbon," respectively. Life had nothing to do with it.
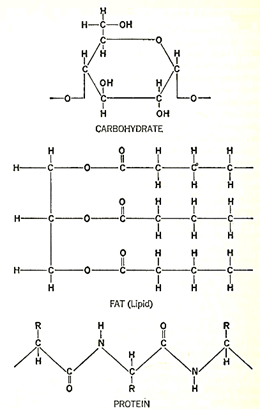
Figure 2. The chemical formulas for the three classes of organic substances of which all things are composed: carbohydrate, lipid (fat), and protein. The carbohydrate is a chain of six-carbon sugar units, only one unit of which is shown. The fat in this illustration is palmitin, one of the commonest, and consists of the glycerol atoms at the left and a long chain of fatty
And yet there was considerable room for the vitalists to retreat. The organic compounds formed by nineteenth-century chemists were relatively simple ones. There existed in living tissue many substances so complex that no nineteenth-century chemist could hope to duplicate them.
These more complex compounds fell into three general groups, as the English physician, William Prout (1785-1850), was the first to state, specifically, in 1827. The names we now give the groups are "carbohydrates," "lipids," and "proteins," The carbohydrates (which include sugars, starch, cellulose, and so on) are made up of carbon, hydrogen and oxygen only, as are the lipids (which include fats and oils). The carbohydrates, however, are relatively rich in oxygen, while the lipids are poor in it. Again, the carbohydrates are either soluble in water to begin with or are easily made soluble by the action of acids, whereas the lipids are insoluble in water.
The proteins, however, were the most complex of these three groups, the most fragile, and, seemingly, the most characteristic of life. Proteins contained nitrogen and sulfur as well as carbon, hydrogen, and oxygen, and, though usually soluble in water, coagulated and became insoluble when gently heated. They were at first called "albuminous substances," because a good example was to be found in egg white which, in Latin, is called "albumen." In 1838, however, a Dutch chemist, Gerard Johann Mulder (1802-80), recognizing the importance of the albuminous substances, coined the word "protein" from Greek words meaning "of first importance."
Throughout the nineteenth century, the vitalists pinned their hopes, not on organic substances generally, but on the protein molecule.
The developing knowledge of organic chemistry also contributed to the evolutionary concept. All species of living things were composed of the same classes of organic substances: carbohydrates, lipids, and proteins. To be sure, these differed from species to species but the differences were minor. Thus, a palm tree and a cow are extremely different creatures, but the fat produced from coconuts and from milk are different in only trivial ways.
Furthermore, it became clear to chemists of the midnineteenth century that the complicated structure of carbohydrates, lipids, and proteins could be broken down to relatively simple "building blocks" in the course of digestion. The building blocks were identical for all species and only the details of combining them seemed different. One creature could feed upon another widely different one (as when a man eats a lobster or a cow eats grass) because the complex substances of the food are broken down to the building blocks held in common by eater and eaten; and these building blocks are absorbed and then built up again into the complex substances of the creature who feeds.
From the chemical standpoint, then, it would seem that all life, however various in outer appearance, is one. If this is so, then evolutionary changes of one species to another would seem to be mere matters of detail; and to require no truly fundamental shift. This view increased the plausibility of the evolutionary concept even if, in itself, it did not establish that concept.
Date added: 2022-12-11; views: 714;
