Major Constituents of Seawater
Table 4-1 lists the major constituents dissolved in seawater. They comprise 99.9 percent of the elements dissolved in seawater. These major constituents exist largely as hydrated free ions, but they can exist in other forms Small amounts of them form ion pairs because of electrostatic attraction between highly charged ions even in the presence of water dipoles.
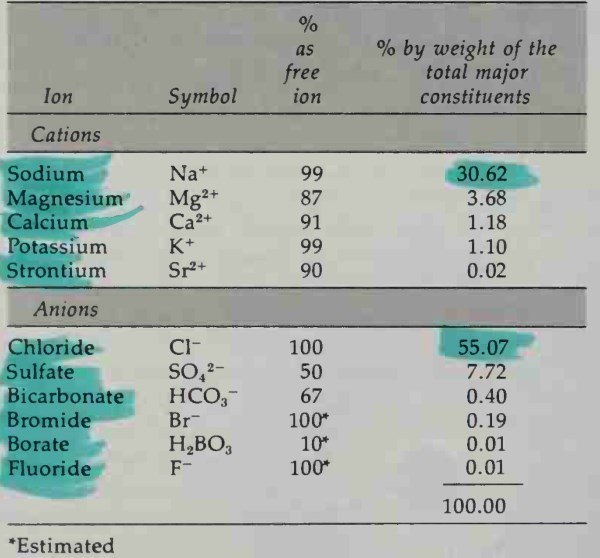
Table 4-1. The Major Constituents of Seawater
The sulfate ion, for example, forms ion pairs with magnesium, calcium, strontium, and other divalent ions; consequently, only 50 percent of it exists as free ions.
Some compounds dissociate only slightly. In seawater, undissociated boric acid molecules predominate (90 percent) over borate ions (10 percent). On the other hand, because fluoride and bromide can form complexes with metal ions, we can expect that these complexes also exist in seawater. Strontium, however, is chemically much like calcium, so it probably exists largely as hydrated free ions.
Trace Elements. Thе rest of the elements dissolved in seawater are present in concentrations of less than 1 part per million. Table 4-2 lists the trace elements in the order of their abundance in seawater. Some trace elements form free ions and ion pairs, but most of these elements exist as organic complexes and, to a lesser extent, as hydroxide, chloride, and possibly fluoride complexes.
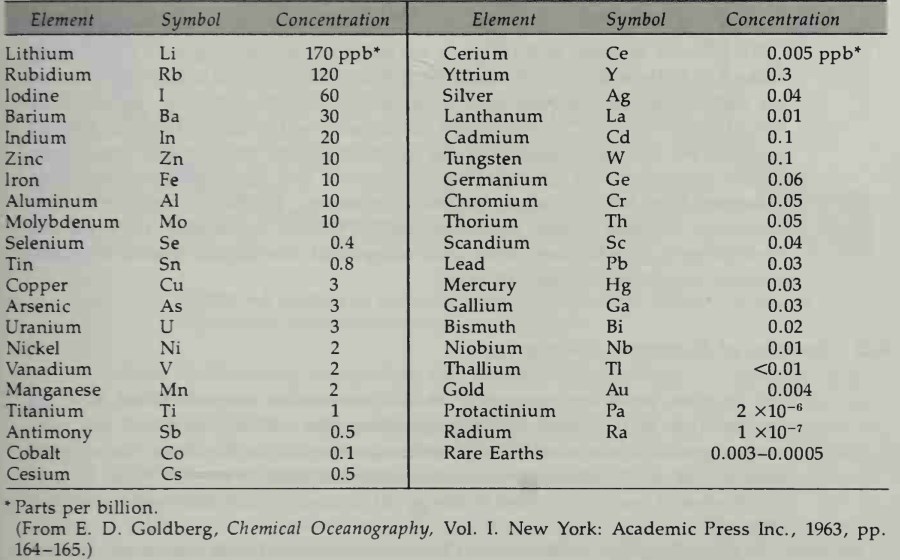
Table 4-2. Concentration of Trace Elements in Seawater Exclusive of Nutrients and Dissolved Gases
The importance of trace elements in seawater became apparent after early attempts to prepare artificial seawater for marine aquaria failed. Even though the major and nutrient ion concentrations could be adjusted correctly, the trace element impurities in the main ingredients caused an upset in the trace element ion concentrations. This water often would not support life, so it seemed reasonable to assume that some trace element ions are important to the biology of the sea.
Nutrient Elements and Organic Compounds. Nutrient elements exist as nitrate (N03-), phosphate (P043-), and silicate (Si03-) ions. As the name implies, the nutrient ions are the fertilizers of the sea. Although they are present in small quantities, the nutrient ions are important because they are necessary for plant growth. Approximate concentration levels are given in Table 4-3. Their importance will be discussed in Chap. 13, which introduces biological oceanography.
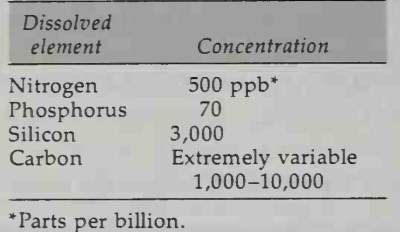
Table 4-3. Average Concentrations of Nutrient Ions Dissolved in Seawater
Also found in small quantities in seawater are various organic compounds that occur both as dissolved molecules and as colloids. These organic substances are carbohydrates, proteins and their decomposition products, lipids (fatty substances), vitamins, auxins (plant hormones), and humic substances formed by complex reactions involving the decomposition products of organisms.
Humic substances are complicated compounds that have been isolated from seawater; they are also called "yellow substances." Fatty acids are particularly important because, upon incorporation in bottom sediments, they rapidly form kerogen, a relatively unreactive compound that slowly yields the compounds comprising crude petroleum.
Date added: 2024-04-08; views: 803;
