Excitatory Amino Acids
Several naturally occurring amino acids possess neuroexcitatory properties and may be transmitters. A large amount of aspartate (-3 mmol g-1 wet weight) is present in the brain, as are small amounts of other excitants, including cysteine sulfinic acid and quinolinic acid.
However, current evidence favors glutamate as the transmitter used by nearly all excitatory pathways in the mammalian central nervous system. These pathways include many that are known to be involved in responses to stress, such as the outflow from the hippocampus to the hypothalamus. Therefore, only glutamate mechanisms are covered here.
Synthesis and Release. The cerebral cortex and other higher brain centers contain more glutamate than any other part of the body, approximately 12 mmol g-1 wet weight. This is more than twice the glutamate content of the spinal cord and much higher than the glutamate content of the peripheral organs. Immunocytochemical labeling methods show an excess of glutamate in excitatory neurons and their axon terminals. The terminals contain small, clear, round synaptic vesicles.
The postsynaptic element is usually a dendritic spine with a broad postsynaptic density. The postsynaptic density contains glutamate receptors and their associated signaling molecules. Approximately 60-70% of all synapses in the brain appear to be glutamate synapses (Figure 1).
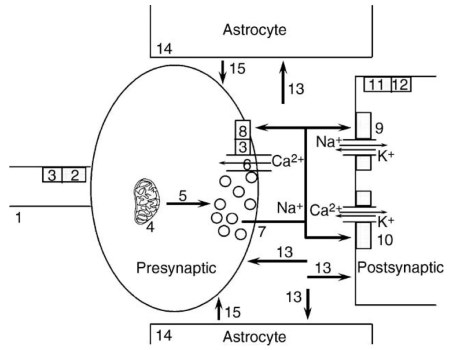
Figure 1. Diagram of a glutamate synapse. (1) Presynaptic action potential. (2) Type II metabotropic receptor. (3) Go, Gi. (4) Glutaminase. (5) Vesicular transport. (6) Voltage-dependent Ca2+ channel. (7) Exocytotic release. (8) Type III metabotropic receptor. (9) AMPA receptor. (10) NMDA receptor. (11) Type I metabotropic receptor. (12) Gq. (13) Plasma membrane transport. (14) Glutamine synthetase. (15) Diffusion and terminal uptake of glutamine
Glutamate can be synthesized by at least six different metabolic routes. However, most of the glutamate that is used as a transmitter is synthesized from glutamine. This biosynthetic process involves cooperation between nerve terminals and the adjacent astrocytes. Glutamate is taken up by astrocytes and converted to glutamine by glutamine synthetase, an enzyme expressed by glia but not by neurons. The glutamine synthetase reaction requires ATP.
Astrocytic end feet, enriched in glucose transporters, cover virtually all capillary walls in the brain. Glutamate transport into the astrocytes stimulates glucose uptake by these cells. Glutamine produced by the astrocytes diffuses into the extracellular space and is taken up by the nerve terminals. Glutamate nerve terminals are enriched in phosphate-stimulated glutaminase, a mitochondrial enzyme that hydrolyzes glutamine to glutamate.
This synthetic pathway is referred to as the glutamate-glutamine cycle. The glutamate-glutamine cycle provides a mechanism for coupling glutamate transmission with glucose use. The energy demands of this cycle account for approximately 85% of total brain glucose use!
The expression of vesicular glutamate transport determines that a particular synaptic terminal will release glutamate. The vesicular transporters are very specific for glutamate and exclude even closely related amino acids, such as aspartate. Transport is driven by an inside-positive vesicular membrane potential and a pH gradient, which are both created by vacuolar H+-ATPase activity. Three cDNAs that encode distinct vesicular glutamate transporters have been cloned.
The transporters are expressed by different neuronal populations. Two of the vesicular transporters were originally identified as inorganic phosphate transporters located on the plasma membrane of the nerve terminal. It is now known that the transporter is incorporated into the vesicular membrane in an orientation that allows for the inward transport of glutamate.
After exocytosis, the transporter is transiently incorporated into the plasma membrane in the opposite conformation (inside-out relative to the synaptic vesicle). In that conformation, it can transport inorganic phosphate into the terminal.
Glutamate is released on nerve terminal depolarization by Ca2+-dependent exocytosis. In the mature brain, the opening of P/Q-type Ca2+ channels in the nerve terminal membrane provides the bulk of the Ca2+ required to drive this process.
Glutamate can regulate its further release by feeding back onto terminal autoreceptors. Glutamate release is also regulated by the previous activity of the synaptic terminal, by other transmitters that may be present locally in the extracellular fluid, and by products of cell metabolism, such as adenosine and arachidonic acid.
Date added: 2024-06-21; views: 469;
