Amino Acid Arrangement
This, however, was still not enough. After all, chemists are interested not only in the number of atoms in an ordinary compound, but in their arrangement as well; and so it is with the amino acids in protein molecules.
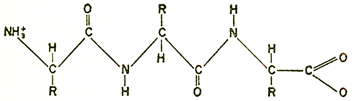
R-Side Chains
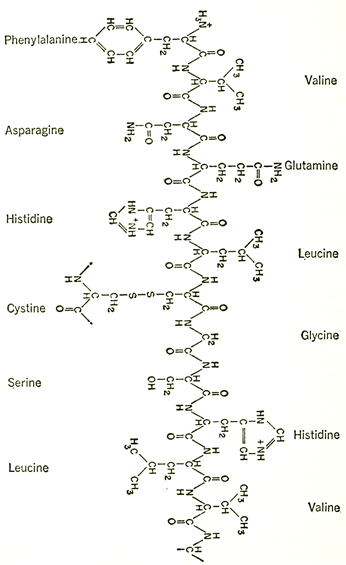
Chemical formulas showing the complex structure of a protein. Above is a portion of one of two peptide chains which form the protein molecule of insulin. The peptide backbone is repeated along the center of the chain and a few of the animo acids are shown linked in as side chains. On the facing page is a portion of the peptide chain which forms the backbone of a protein. R represents the amino acid side chains.
The question of arrangement is a difficult one, though. With even a few dozen amino acids in a molecule, the number of possible different arrangements is astronomical, and with 500-plus amino acids present (as in the molecule of hemoglobin, which is only of average size for a protein) the different arrangements possible must be represented by a number with over six hundred digits! How might one choose the one correct order out of so many possibihties?
With paper chromatography, the answer proved easier than might have been expected. Working with the insuhnmolecule (made up of but some fifty amino acids), the English biochemist, Frederick Sanger (1918- ), spent eight years working out the method. He broke down the insulin molecule partway, leaving short chains of amino acids intact. He separated these short chains chromatographically and identified the amino acids making up those chains, as well as the order of arrangement in each. This was not an easy task, since even a four-unit fragment can be arranged in twenty-four different ways, but it was not a completely formidable task either. Slowly, Sanger was able to deduce which longer chains could give rise to just those short chains he had discovered and no others. Little by little, he built up the structure of longer and longer chains until, by 1953, the exact order of the amino acids in the whole insulin molecule had been worked out.
The value of the technique was demonstrated almost at once by the American biochemist, Vincent du Vigneaud (1901- ). He applied the Sanger technique to the very simple molecule of "oxytocin," a hormone made up of only eight amino acids. Once their order was worked out, the fact that there were only eight made it practical to synthesize the compound with each of the amino acids in the proper place. This was done in 1954, and the synthetic oxytocin was found to be exactly like the natural hormone in all respects.
Both Sanger's feat of analysis and Du Vigneaud's feat of synthesis have been repeated on a larger scale since. In i960, the arrangement of the amino acids in an enzyme called "ribonuclease" was worked out. The molecule was composed of 124 amino acids, two and a half times as many as the number of amino acids in the insulin molecule. Furthermore, fragments of the ribonuclease molecule could be synthesized and studied for enzymatic effectiveness. By 1963, it was discovered in this way that amino acids 12 and 13 ("histidine" and "methionine") were essential for the action of the molecule. This was a long step toward determining the exact manner in which a particular enzyme molecule performed its function.
Thus, as the mid-century progressed, the protein molecule was gradually being tamed by the advance of knowledge.
Date added: 2022-12-11; views: 866;
