Evidence Supporting Gender Differences in Stress Responses
Gender Differences in Adrenal Function. The early work of Hans Selye described adrenal gland function and its role in homeostasis. This work also provided the first compelling evidence for potential sex differences in stress responses. In supporting the observations of Selye, many studies showed that the adrenal gland is larger in females than in males, even when corrected for sex differences in body weight.
Correspondingly, the rate of hormone secretion by the adrenal gland differs between males and females. Moreover, some studies in rodents reported that females show a greater diurnal fluctuation in corticosterone or cortisol, the main steroid hormone secreted by the adrenal cortex (corticosterone in the mouse and rat, cortisol in the hamster and human; Figure 1).
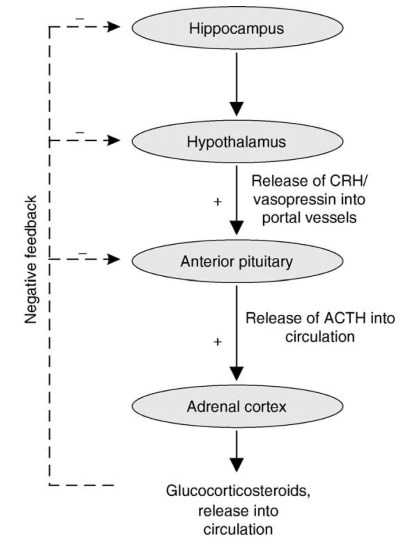
Figure 1. Schematic representation of the hypothalamic-pituitary-adrenal (HPA) axis. The positive feedback portion of the HPA starts at the level of the paraventricular nucleus of the hypothalamus. Neurons residing in this nucleus release corticotropin releasing hormone (CRH) and vasopressin into the hypophysial portal vessels to the anterior pituitary to stimulate the synthesis and release of ACTH.
This in turn stimulates both synthesis and release of adrenal glucocorticoids into the general circulation to elicit an appropriate stress response. The presence of increased levels of glucocorticosteroids in turn induces an inhibitory effect (i.e., negative feedback) on the HPA axis, which is thought to be mainly mediated through glucocorticoid-responsive neurons in the hippocampus
Similarly, the adrenal response in secreting these glucocorticoid hormones following stressful, or perceived stressful, situations such as exposure to noxious fumes, exposure to a novel environment, immobilization, or a brief electric shock (see Glucocorticoid Negative Feedback, Glucocorticoids, Effects of Stress on, Glucocorticoids - Adverse Effects on the Nervous System, Hippocampus, Overview) resulted in peak elevations in adrenal glucocorticoid hormone levels that were 1.5-2 times greater in females than in males.
In adult animal models, sex differences in adrenal function appear to be relatively confined to glucocorticoids because gender differences in other adrenal cortex-derived steroid hormones, such as progesterone and androgen, have not been reported in the literature.
Androgen and progesterone are secreted predominantly by the gonads at relatively high levels. Therefore, gonad-derived steroids may mask any sex differences in adrenal-derived steroids, which may underlie the absence of these types of studies in the literature.
Sex Differences in Neuroendocrine Function. Pituitary hormone secretion The sex difference in adrenal function is in part due to sex differences in the neuroendocrine axis that regulates adrenal hormone secretion. This axis has been termed the hypothalamic-pituitary-adrenal (HPA) axis because of the interaction of each of these tissues in regulating one another’s secretory activity (Figure 1).
Similar to sex differences in the adrenal glucocorticoid release, adrenocorticotropic hormone (ACTH) is also released in a sexually dimorphic fashion. ACTH is a hormone secreted by the anterior pituitary gland into the general circulation, which in turn stimulates adrenal synthesis and secretion of glucocorticoids.
Animal studies also demonstrated that the increases in circulating ACTH levels following physical or psychological stressors are greater and more prolonged in females than in males. An enhanced ACTH response in female rodents seems to depend on the hypothalamic peptide called corticotropin releasing hormone (CRH), suggesting that the sex difference in ACTH response may, in part, be the result of a sex difference in anterior pituitary responsiveness to CRH.
Hypothalamic function. The secretion of ACTH from the anterior pituitary is controlled by hormones that are synthesized in neurons residing in the paraventricular nucleus of the hypothalamus (PVN). These hormones are released into the hypophysial portal vasculature via the median eminence (Figure 1).
The most important releasing factors for ACTH appear to be CRH and vasopressin. Both CRH and vasopressin are potent ACTH secretagogues, and vasopressin potentiates the ACTH-releasing activity of CRH several-fold. Animal studies have shown that the PVN in females contains more CRH neurons than in males. Moreover, females exhibited a more pronounced diurnal variation in hypothalamic CRH immunoreactivity than males. These data are consistent with physiological data demonstrating a greater activation of the HPA axis in females.
Studies have demonstrated sex differences in the release of vasopressin following a stressor. Indeed, vasopressin was released in female rats following immobilization stress, whereas this was not the case in male rats. Although, vasopressin immunoreactivity in the PVN is not sexually dimorphic, the presence of a sex difference in the functional sensitivity of vasopressin cells in the PVN to stressors may help explain sex differences in neuroendocrine responses.
Negative feedback regulation. The stimulatory limb of the HPA axis (CRH, vasopressin, and ACTH) is kept in check by continuous feedback inhibition by circulating glucocorticoid hormones. Thus, it has been hypothesized that the intensity of this negative feedback action may be an underlying factor in the sexually dimorphic secretion of adrenal glucocorticoid hormones.
The negative feedback glucocorticoids is mediated by two receptor types - mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) - that reside in target neurons in the hypothalamus, hippocampus, and anterior pituitary gland, as well as other brain areas. Both receptors appear to be involved in differing aspects of the negative feedback regulation.
Because of their high affinity for glucocorticoids, MRs are thought to regulate basal hormone secretion, whereas GRs, which exhibit a lower affinity for glucocorticoids, are thought to return stress-responsive elevations in glucocorticoids back to baseline. Assuming that the number of receptors accurately reflects the sensitivity of a tissue for a hormone, it is logical to conclude that sex differences in the HPA axis may result from a sex difference in the number of receptors in a given cell.
A greater negative-feedback sensitivity would be indicated by more receptors and consequently result in lower basal and stress-activated hormone secretion. On the other hand, lower levels of receptors would indicate weaker negative feedback and thus result in higher basal and stress-responsive hormone secretion. This hypothesis has been supported by sex differences in the concentration and function of MRs and GRs.
Overall, females tend to have lower levels of MRs and GRs in many brain regions, including the hypothalamus and hippocampus, which is consistent with the pattern found in other tissues, such as the thymus and liver. Given similar levels of hormone, GR- or MR-mediated functions (e.g., autoregulation of the receptors) was reported to be less sensitive to glucocorticosteroid modulation in females than in males.
Sex Differences in Behavioral Responses to Stress. Sex differences in behavioral responses to stress have been well-documented in humans and animals. Laboratory animals have provided much of what we know regarding gender differences in response to stress. When placed in a given stressful situation, animals undergo a series of well-described behaviors that have been interpreted to indicate the degree of fear or anxiety that is being experienced.
Freezing, defecation, and vocalization are a few good examples of these behaviors. Females appear to be less sensitive to stress-associated novel or aversive environments. For example, when placed into a novel environment or an elevated plus maze, female animals show less freezing and defecation than males.
Females also enter a compartment in which they have been previously shocked more readily. In these behavioral paradigms, females also show more activity and exploration, behaviors that have been interpreted as signifying a lesser expression of fear and anxiety. However, when exposed to a variety of chronic stress paradigms, females are found to habituate significantly less than males.
In a well-studied animal model of chronic stress known as learned helplessness, animals are subjected to a repeated inescapable stressor, such as an intermittent tail shock. Subsequently, when allowed to escape, male rats are impaired in their escape performance, whereas female rats do not show this deficit to the same extent.
However, when stressed repeatedly over a long period of time, it appears that males adapt more readily (eventually do not show reduced activity), whereas females fail to adapt over the same time period. It appears that this sex difference in adaptability may be dependent on levels of glucocorticoids because the reduction of the poststress glucocorticoid levels in females to levels attained in males allows the adaptation to repeated restraint. However, these effects are not always observed across studies.
One reason may be related findings from studies showing that both strain and stress exposure during early development are important determinants in the expression of stress-related sex differences in behavior in adulthood.
Date added: 2024-07-10; views: 502;
