Basic Mechanisms of Sexual Differentiation of Neural Function
Sexual differentiation of the brain occurs as a result of the presence of gonadal steroid hormones acting during either development or adulthood (Figure 2). The actions of gonadal steroid hormones on the brain have been classified as being either organizational or activational. Organizational actions are permanent changes in brain structure or function that occur during development.
Generally, these permanent effects of gonadal steroid hormones are greatest during a defined critical window of opportunity, usually during gestation or early postnatal life. The removal of gonadal steroid hormones after this critical period does not alter the organization or function of the brain appreciably.
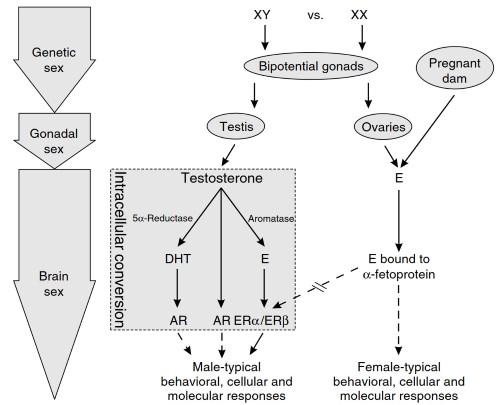
Figure 2. Schematic representation of the gonadal steroid hormones involved in the sexual differentiation of the vertebrate brain during development. According to this scheme, a-fetoprotein sequesters circulating estrogen, thereby preventing the defeminization (or masculinization) of the male or female brain. Therefore, the brain is thought to be initially programmed to develop in the female direction.
However, α-fetoprotein is bypassed in the male brain because circulating testosterone is converted intraneuronally into estrogen by the enzyme aromatase, thus enabling estrogen to defemininize (or masculinize) the male brain, which is mediated through estrogen receptors. AR, androgen receptor; DHT, 5α-dihydrotestosterone; E, estrogen; ER, estrogen receptor
In contrast, the activational effects of gonadal steroid hormones are those that occur transiently, in direct response to the presence of a gonadal steroid hormone. Indeed, the removal of gonadal steroid hormones from the circulation, through castration or ovariectomy, results in a loss of effect. The activational effects of gonadal steroid hormones can occur throughout life, but are generally thought to have a maximal influence on gender differences during adulthood.
The literature regarding sexual differentiation of the brain suggests that the mammalian brain is an undifferentiated or perhaps neutral state and that, in response to normal circulating levels of testosterone (from the fetal male gonad), it develops toward the male direction. In contrast, if testosterone is low or absent, the brain develops toward the default mode, which is female (see Figure 2).
Although testosterone or its androgenic metabolite DHT may play a role in the masculinization of the brain, through their intracellular androgen receptor, in rodent models the functional gonadal steroid hormone for the organizational sexual differentiation of the brain is considered to be estrogen (Figure 2).
This steroid hormone is produced intracellularly as a metabolite of testosterone due to the actions of the enzyme aromatase. The defeminizing effects of estrogen on the developing brain are mediated by estrogen receptors (ERs), although the exact role of each of the two forms of ERs, ERα and ERß, is not known.
Circulating estrogen from the mother or the developing ovary of the female is bound to a liver-derived protein called a-fetoprotein, which is present in high levels during perinatal development. Consequently, circulating estrogens are sequestered and cannot defeminize (or masculinize) the developing male or female brain. In contrast, defeminization (or masculinization) by estrogen can occur in the male brain because the estrogen-sequestering abilities of α-fetoprotein are circumvented due to its intracellular origin through the aromatization of testosterone.
In rodents, the high levels of α-fetoprotein decrease during the second and third weeks of life, which coincides with increasing levels of unbound estrogens in the circulating, thus allowing the organizational and activational effects of estrogens to occur at puberty and in adulthood.
Organizational Effects of Gonadal Steroid Hormones on Stress Responses. Gonadal steroid hormones may act during early development to permanently organize adult responses to stress. This is best illustrated by examining the effects of perinatal hormone removal and replacement on the development of the adult pattern of behavior in response to stress.
An example is the earlier described sex difference in the performance of the elevated plus maze and open-field test, in which female rats showed more activity and were less anxious than male rats. Indeed, the activity of an adult male rat that was castrated postnatally (a procedure that removes circulating levels of testosterone and its associated metabolites, such as estrogen) is similar to that of a female rat.
The castration of male rats up to 21 days after birth prevents the development of the sex difference in activity. In contrast, neither the castration of adult males nor estrogen or testosterone replacement affects the sex difference in the level of activity. Similarly, the treatment of female rats with testosterone or estrogen up through 4 weeks after birth, but not in adulthood, results in a more malelike activity pattern.
These studies indicate a critical period of sensitivity for the organizing actions of gonadal steroid hormones; moreover, they also show that sex differences in the activity level do not arise until after puberty.
Few studies have examined the occurrence of similar organizational effects of sex steroids on neuroendocrine responses to stress. The neonatal gonadectomy of male rats produces a more female-like hormonal response to stress in adulthood, but much of this may be due to the absence of hormones in adulthood because adult gonadectomy has a similar effect.
On balance, it appears that the predominant effects of gonadal steroid hormones on neuroendocrine stress responses are activational in nature, with organizational influences contributing a much more modest amount to the overall sex difference.
Date added: 2024-07-10; views: 509;
