Glycoconjugates Are Important Mediators of Many Physiological Functions
Glycosylation is a very complex posttranslational modification that plays an important role in the integration of higher organisms. The surface of all cells is covered by oligosaccharide structures covalently attached to proteins and lipids (glycoproteins and glycolipids). Escalation in the extent and diversity of protein glycosylation correlates with the appearance of multicellular life, and apparently most of the interaction between cells and their surroundings include carbohydrate-protein recognition.
An essential difference between proteins and the oligosaccharide structures attached to them is the fact that the carbohydrate part is not encoded in the DNA (Figure 1). Although genes unequivocally define the structure of each protein, there is no template for attached oligosaccharides. This makes them inherently sensitive to all changes within the cell, and glycan structures that are being produced at any individual moment actually mirror all relevant past events in the cell.
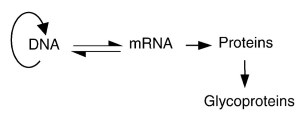
Figure 1. Central dogma of molecular biology amended with the biosynthesis of glycoproteins. Contrary to proteins, which all have their templates in the DNA, the carbohydrate structures attached to glycoproteins are not defined by genes. Their exact structures are determined by the regulation of expression, intracellular localization, and activity of various glycosyltransferases
In addition, the evolution of carbohydrate structures is much faster than the evolution of proteins, and often in different species we see significant differences in carbohydrate structures attached to nearly identical protein or lipid backbones.
Glycan parts of glycoconjugates are being synthesized by GTs that act in a sequence, and each enzyme transfers specific monosaccharide to a specific acceptor contributing to the final glycan structure (Figure 2). It is estimated that over 300 specific enzymes (glycosidases and GTs) are involved in the synthesis of carbohydrate structures on glycoconjugates, and the whole process is very complex and energy expensive for the cell.
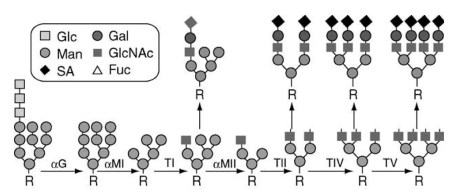
Figure 2. Schematic representation of processing of N-linked glycans. N-linked glycans are being synthesized by sequential action of numerous glycosyltransferases and glycosidases that add or remove specific monosaccharides to a growing oligosaccharide chain
Defects that disturb early phases of glycoconjugate synthesis are incompatible with multicellular life, whereas mutations in the end modifications of oligosaccharides result in specific physiological alterations. Because oligosaccharide structures are not encoded in genes, the final structure of a glycan is determined by the current cellular repertoire of expressed GTs (the expression, intracellular localization, and regulation of specific GTs that is commonly referred to as the glycosylation phenotype).
At the level of the individual molecule, from the structural point of view, there is no significant difference between the protein and carbohydrate parts. Glycoprotein is an entity composed of a protein and a carbohydrate part, and both parts have structural and functional roles that are specific to a given protein.
Due to very high structural variability and the lack of adequate methods to analyze them, the carbohydrate parts of glycoconjugates were ignored by the most of the scientific community until very recently. However, thanks to the development of novel methods, in the last decade there has been an exponential increase in the knowledge about the physiological roles of carbohydrate structures.
Now it is known that glycan part of glycoconjugates are crucial for many physiological processes from fertilization and development to regulation of hormonal activity and the formation of memory. Recent evidence has established differential glycan processing as a novel regulator of protein function, and the speculation that the invention of glycosylation was the key evolutionary step that enabled the development of multicellular organisms appears to be correct.
Date added: 2024-08-26; views: 381;
