Stress Affects the Activity of Glycosyltransferases
The carbohydrate parts of glycoconjugates are synthesized by sequential action of numerous GTs, enzymes that are specific for both the structure of the glycoconjugate acceptor and the monosaccharide that is being added. The appearance of novel or altered glycoconjugate structures in stress indicates that stress should somehow affect the activity of GTs, but the only GTs whose relation with stress have been studied up to now are the sialyltransferases (enzymes that transfer sialic acids to glycoproteins).
For some time it has been known that corticosteroids can change the activity of sialyltransferases in vitro. Breen and colleagues have shown that corticosteroids and other hormones from the adrenal gland significantly influence the activity of sialyltrans-ferases in vivo. Adrenalectomy and the subsequent administration of corticosterone and/or aldosterone significantly influence the activity of sialyltransferases in various rat tissues.
Whereas sialyltransferases in some tissues such as the kidney are apparently not influenced by adrenalectomy or by the addition of steroid hormones, sialyltransferases in the liver are under the negative control of corticosteroids. Adrenalectomy results in the increased activity of sialyltrans-ferases in the liver that cannot be reverted to normal values by the administration of dexamethasone.
Enzymes that perform the same function in the brain react to same hormonal signals in the exactly opposite way. Adrenalectomy and the consequential lack of circulating corticosteroids lead to the decrease of total sialyltransferase activity in the brain. The subsequent administration of exogenous corticosteroids exhibits regional specificity, with the enzyme activities in the cortex, cerebellum, and brain stem being stimulated by both dexamethasone and aldosterone and enzyme activity in the hippocampus being stimulated only by aldosterone.
Total sialyl-transferase activity in some tissue does not represent the activity of a single enzyme but the sum of the activities of all enzymes that transfer sialic acids. Breen and colleagues also studied the effects of corticosteroids on two individual enzymes, α(2,3)- sialyltransferase and α(2,6)-sialyltransferase. Both enzymes transfer sialic acids, but they link them to different carbon atoms on the preceding sugar in the carbohydrate structure.
As shown by Dabelic and colleagues in 2004, immobilization stress affects sialyltransferase activity in different rat tissues. Acute and chronic stress have different effects, but, even more interesting, the same type of stress has opposite effects on sialyltransferase activity in different tissues. In general, stress induces sialyltransferase activity in extraneural tissues and suppresses the activity of the same enzymes in most brain tissues.
The fact that same enzymes respond differently to the same hormonal signals depending on their cellular environment exemplifies the fact that the molecular setup of the targeted cell, and not hormonal signal by itself, is the decisive player in determining the direction and consequences of the stress response.
Model of Glycobiology of Stress. At the moment only several fragments of the glyco- biological mechanisms involved in the physiological response to psychological stress are known, but the complete picture is slowly emerging (Figure 4). Stress causes numerous changes in the circulating hormones, and many molecular details of this process are known. It is also known that corticosteroids affect the activity of at least one GT both in vitro and in vivo.
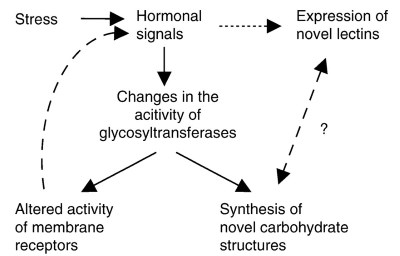
Figure 4. Hypothetical model of the glycobiology of stress. Hormones mediate numerous stress-associated changes, and among them alter the activity of different glycosyltransferases. Changed activity of glycosyltransferases results in the appearance of different carbohydrate structures on glycoproteins, which could either present a novel structures with potential to interact with specific endogenous lectins or modify the activity of various membrane receptors.
Although this has yet to be proven, it could be one of the mechanisms explaining why prolonged stress has different effects than acute stress. In the same time, it is known that stress is associated with changes in composition and activity of endogenous lectins, but the exact mechanisms linking stress and changes in lectin activity are still not known
The altered activity of GTs results in different carbohydrate structures attached to glycoproteins, and these changes have been demonstrated both in humans and in experimental animals. A change in the carbohydrate structures attached to a glycoprotein is a well-established way to change its structural and functional properties, and recently this was shown to be one of the mechanisms that control the activity of membrane receptors.
Although this type of glycosylation-mediated receptor modulation in stress still has to be proven, it is a very interesting hypothesis. On the other hand, new glycoconjugate structures could also represent novel signals on the cell surface that could alter the interaction of the cell with neighboring cells in a process analogous to the selectin-mediated adhesion of lymphocytes. Stress is also known to be associated with the appearance of novel lectins, but the exact mechanism of this process is not known.
These lectins could be receptors for either novel or normal glycoconjugate structures, translating their structures into molecular functions. Although most of this is still speculative, it is hoped that more will soon be known about the molecular role of glycoconjugates, their lectin receptors, and GTs in the physiological response to psychological stress.
Date added: 2024-08-26; views: 393;
