Lectins in Stress
Some glycans on glycoconjugates have only structural roles, but some also take part in specific recognition processes. One of the major mechanisms by which glycoconjugates perform their molecular functions is the interaction with their specific molecular receptors, lectins.
Hundreds of endogenous lectins function as physiological receptors for oligosaccharides that interpret molecular information encoded in glycans. They take part in numerous physiological processes including folding, intracellular transport, fertilization, regulation of the inflammatory response, and brain plasticity.
The best-known example of lectin function is inflammation, in which the interaction between lectins called selectins and carbohydrate structures on glycoproteins represents the first decisive step leading to the adhesion of circulating lymphocytes to the endothelium at the site of inflammation. Carbohydrate-lectin interactions are very interesting because they can be inhibited with small oligosaccharides that are generally nontoxic and, using modern carbohydrate cycling technologies, are relatively inexpensive to prepare for therapeutic purposes.
The first indications that lectins might change in stress came from a study of a phenomenon only remotely associated with stress. Bardosi and colleagues were analyzing the influence of prolonged anesthesia on mannose receptor and some other lectins on murine peripheral blood polymorphonuclear leukocytes. They demonstrated reduced expression of mannose receptor and changes in the expression of receptors for several other sugars, indicating that prolonged anesthesia affects the regulation of lectin expression.
Evidence that psychological stress can influence lectins came from studies of Lauc and colleagues on lectins in livers of rats exposed to immobilization stress. Immobilization stress was found to influence galectin-3, a galactose-specific lectin, so that it binds to another nuclear lectin CBP67 (67-kDa carbohydrate binding protein). The formation of the complex from CBP67 and galectin-3 resulted in the binding of the galactose-specific galectin-3 to the glucose- affinity column, for which it shows no affinity under normal conditions.
This is a very interesting effect because galectin-3 is involved in mRNA splicing and changes in its functions might have profound effects on the whole cell. However, whether this binding was mediated by protein-protein interactions or through lectinlike binding of galectin-3 to galactose residues on carbohydrate structures attached to CBP67 (as well as other details of this interaction) is not known.
Galectin-3 is a versatile galactoside-binding lectin that has been implicated in numerous cellular functions. It also appears to be affected by stress and, interestingly, different types of stress have exactly opposite effects on its expression. As shown by Dumic and colleagues in 2000, whereas exposure to UV light or transfer to in vitro conditions induces galectin-3 in cultured cells, immobilization stress in vivo results in a decrease in galectin-3 in mouse spleen and liver.
The regulation of galectin-3 expression involves transcription factor nuclear factor (NF)-kB, which connects galectin-3 with corticosteroids (and CRH) and places its expression downstream from hormonal signals in the stress-response pathway.
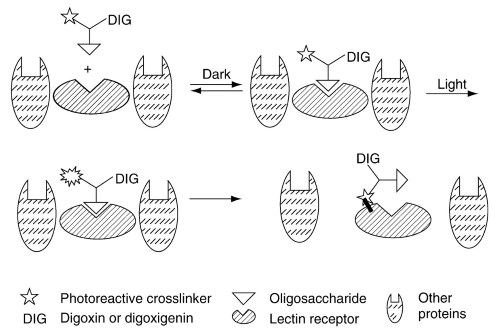
Figure 3. Photoaffinity method for the detection of lectins. Photoaffinity glycoprobes containing target carbohydrate structures are incubated with biological samples to allow the formation of noncovalent complexes between the probe and lectin receptors in the sample.
Illumination activates the photoreactive cross-linker, and it forms covalent bonds with neighboring molecules, mostly lectin receptors. The result is a lectin with a covalently incorporated digoxin tag (DIG) that can be easily identified with labeled antibodies against digoxin
A major problem in studying changes of lectin activity in different physiological processes is the lack of adequate methods for measuring lectin activity in complex biological samples. A new method (Figure 3) using photoaffinity glycoprobes labeled with digoxin was recently developed, and it is hoped that it will enable easier identification of changes in lectins in different diseases. Until now, only one new lectin was found to appear in stress. It was found to bind to glucose-containing glycoprobes, and according to the electrophoretic mobility of the protein isolated from rat liver named CBP33.
Date added: 2024-08-26; views: 390;
