Disinfection Kinetics
Ideally, microorganisms of the same species are distinct units equally susceptible to a single type of disinfectant, both microorganisms and the disinfectant are evenly dispersed, and the disinfectant remains unchanged in chemical composition and constant in the concentration in water with no interfering substances. When there is uniform dispersion, constant chemical composition, no interfering substances, and equal susceptibility, the rate of disinfection is first order as a function of contact time, disinfectant concentration, and water temperature.
Many studies only present results through reports of log10 or percent reduction and do not demonstrate that first-order kinetics were observed. However, first-order kinetics were confirmed for Staphylococcus sp. when exposed to free chlorine alone and in combination with electrolytically produced silver ions and copper ions in tap water containing urine and bathwater and in well water.
A swimming pool simulation experiment did not release data over time, but indicated first-order inactivation of Legionella pneumophila, Staphylococcus aureus, Staphylococcus sp., Escherichia coli, and Streptococcus faecalis exposed to combinations of copper ions, silver ions, and free chlorine. The disinfection of L. pneumophila was also confirmed to be first order in 100 mL of filtered well water. In addition to bacteria, first-order disinfection rates for the amoeba form of Naegleria fowleri and MS-2 virus in well water were reported for different combinations of free chlorine, copper ions, and silver ions as well. A summary of these values is provided in Table 1 in descending order of inactivation rate (k), for each pathogen reported in the literature.
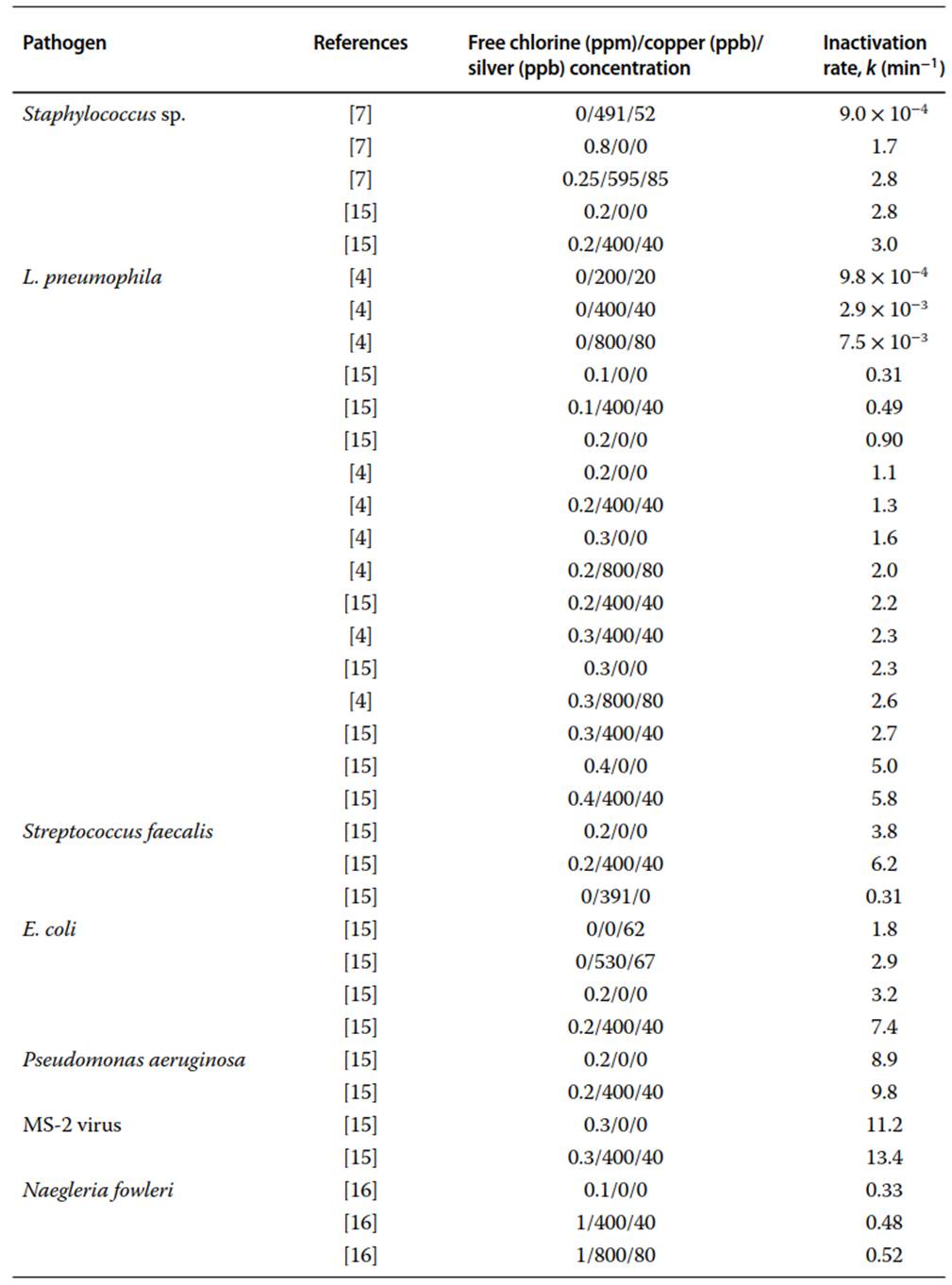
Table1. First-order disinfection rate coefficients reported in the literature for different microbial pathogens at different free chlorine, copper, and silver concentrations
There is little overlap between the type of pathogens tested in the literature as can be seen in Table 1. However, some comparisons can be made. Overall, the concentration of the free chlorine dose added to each system was more influential on the inactivation rates compared to the concentration of silver or copper ions. This trend is consistently seen within individual experiments and when comparing inactivation rates of L. pneumophila from different experiments. In the same trend, it can be observed that copper and silver ions added to a given amount of free chlorine consistently increase the inactivation rates and often were added to a lower dose of chlorine to produce the inactivation rates comparable to a higher dose of free chlorine alone.
With the exception of one experiment, all inactivation rates found in the literature were measured in groundwater at room temperature in various water chemistries. Many studies failed to report water chemistry parameters, making a true comparison of inactivation rates difficult. One study found that the effects of pH and temperature have no appreciable effect on the performance of biocide systems. In contrast, it has also been observed that copper and silver disinfection can be influenced by pH, chlorides, phosphates, and calcium ions.
Another study found that copper and silver release increased with increased water temperatures. However, this association was not statistically significant (disinfection rate (k) = 1.077 and 1.322 in lower temperatures compared to 1.186 and 2.559 for free chlorine and free chlorine in combination with silver and copper, respectively). The reported physiochemical characteristics of the water from the literature are included in Table 2.
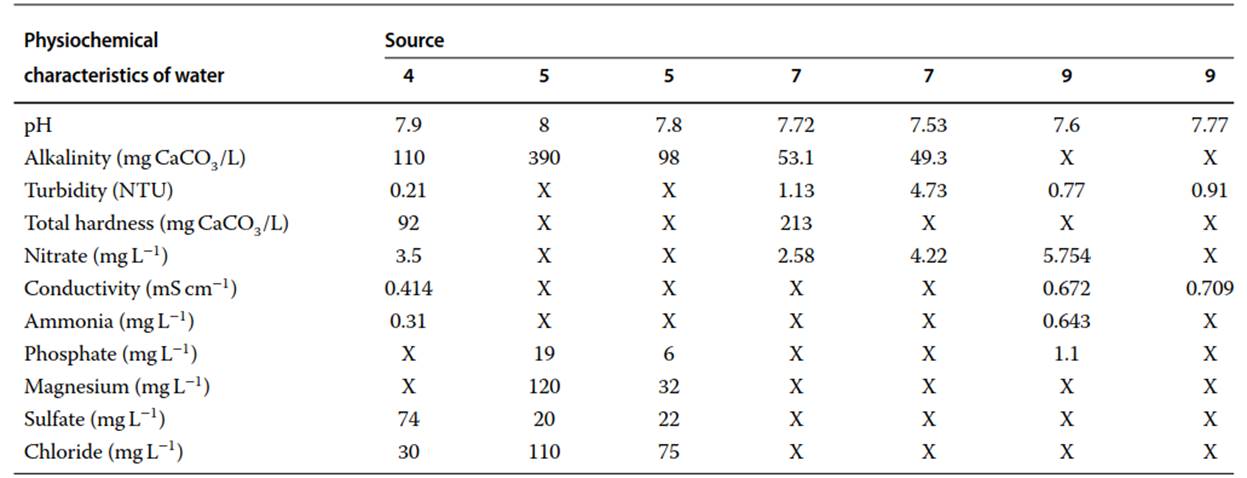
Table2. Physiochemical characteristics of water used in disinfection
In one of the studies included in Table 1, bathwater and urine were added to the experimental apparatus in order to simulate the organic loads from swimmers in pools. The inactivation rates of Staphylococcus sp. determined from this experiment are much lower than the rates reported for well water only. This was due to organic demands from urine and bathwater introduced into the system, which highlights the need to further study the effect of increased organic loads on silver and copper disinfection.
With the exception of this study, this relation has not been previously studied and is vital in order to understand the applicability of silver and copper ions for disinfection in drinking and recreational water treatment. This is particularly important if this technology were to be used as a water treatment method in the developing countries, given that the organic loads of water disinfected immediately after collection from the source and/or stored in the home would be far higher than water reaching the disinfection stage in a conventional wastewater treatment plant.
One advantage of disinfection with silver and copper compared to free chlorine is that chlorine concentrations were shown to decrease dramatically during disinfection. In a continuous flow water system loaded intermittently with bathwater and urine in an outdoor atmosphere, there was no free chlorine detected three to four hours due to organic demands previously discussed, exposure to high temperature, and solar radiation. An identical indoor system observed similar but slower decreases with some residual up to the next morning. In a study of disinfection of various human enteric viruses, values of free chlorine were 79%, 35%, and 29% of original values of 0.94, 0.51, and 0.17 mg L-1 of free chlorine, respectively, after 30 minutes. In the same study, 75% and 44% of electrolytically generated copper and silver remained after 60 days, respectively.
Theoretical first-order disinfection kinetics were easily replicated in experiments for bacteria, N. fowleri, and MS-2 virus over short periods of time. However, an experiment investigating the disinfection of various human enteric viruses in well water by copper, silver, and free chlorine yielded inactivation kinetics that ceased to be truly first order when carried out over longer periods of time. This phenomenon is depicted in Figures 3 a, b.
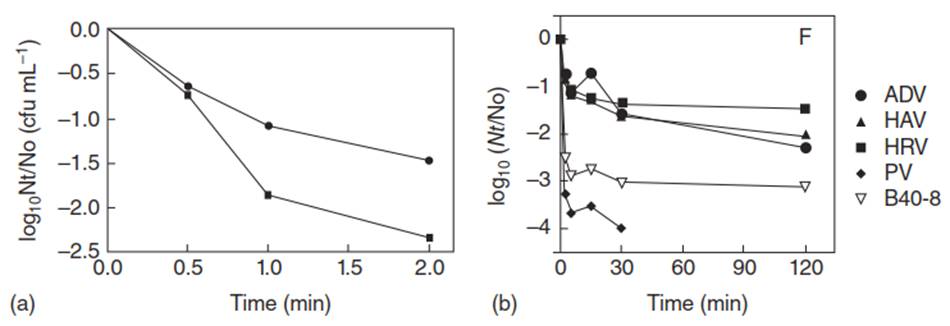
Figure 3. (a) Reduction of Staphylococcus sp. Numbers in water systems after exposure to •, Free chlorine (0.8 mg L-1) ■, copper:silver (595 and 85 µg L-1 copper and silver respectively) and free chlorine (0.25 mg L-1). (b) Inactivation of human enteric viruses by free chlorine (0.2 mg L-1) and copper (700 µg L-1) and silver ions (70 µg L-1) for adenovirus (ADV), hepatitis A virus (HAV), human rotavirus (HRV), poliovius (PV), and Bacteriophage B40-8 (B40-8)
Figure 3a was taken from a disinfection experiment of Staphylococcus sp. in well water over two minutes with 0.8 mg L-1 of free chlorine alone and 0.25 mg L-1 free chlorine in combination with 595 and 85 µg L-1 copper and silver, respectively. Figure 3b displays the disinfection of a variety of human enteric viruses in well water with exposure to 0.2 mg L-1 free chlorine in combination with 700 and 70 µg L-1 copper and silver, respectively. It can be seen that the data in Figure 3a fit a first-order kinetic model.
In Figure 3b, similar doses of free chlorine/silver ions/copper ions are used, but the plots are distinctly concave up. However, if you exclude the early time data, they are approximately linear. This shows that disinfection (k) rates reported in the literature could not be indicative of true performance if the duration of the experiment is not long enough and that further studies should be done on longer term bacterial and amoeba disinfection to better understand and more accurately predict disinfection kinetics in the field.
While the cause of the observed inactivation kinetics is not fully understood, virus aggregates were detected via electron microscopy in test samples treated by disinfection combinations including silver and copper ion.
It has been documented that viruses persist for longer periods of time when they form aggregates as opposed to their presence as individual particles and that divalent cations have been reported to induce virus aggregation. Because of this, it is likely that electrolytically generated Cu2+ ions influenced the disinfection kinetics of the viruses in this study. Unfortunately, data for the disinfection of silver ions or copper ions in combination with chlorine was not tested in the study, so an association between silver and aggregation of these viruses was not determined.
However, the presence of Cu2+ alone cannot account for the bimodal kinetics observed in inactivation curves, as the inactivation kinetics observed for free chlorine alone in these experiments also did not exhibit first-order behavior. A likely explanation is that in the absence of proper evidences, the reported first-order disinfection kinetics in reality are pseudo-first order kinetics, and the data did not fit to a first-order disinfection kinetic behavior could also be due to various environmental factors, such as temperature, light, pH, dissolved oxygen, or halogen demand of the sample, which could affect microorganism susceptibility to silver and the interaction of copper and silver. Disinfection kinetics could have also been affected by well water constituents, which could have caused silver or copper ions to precipitate out of the solution.
Date added: 2025-01-04; views: 515;
