Metabolism. Metabolic Intermediates
The effect of chemotherapeutic agents on insects, weeds, and microorganisms is that of interfering with the pattern of metabolism—sabotage of the organisms' chemical machinery, in other words. The search for such agents is increasingly rationalized by growing knowledge concerning the details of metabolism.
In this respect, the English biochemist, Arthur Harden (1865-1940), led the way. He was interested in the enzymes in yeast extract (the extract which Buchner had shown to be as efficient at breaking down sugar as the yeast cells themselves. In 1905, Harden noted that a sample of extract broke down sugar and produced carbon dioxide quite rapidly at first, but that with time, the rate of activity dropped off. This might seem to be due to the gradual wearing out of the enzymes in the extract, but Harden showed this was not the case. If he added small quantities of sodium phosphate (a simple inorganic compound) to the solution, the enzyme went back to work as hard as ever.
Since the concentration of the inorganic phosphate decreased as the enzyme reaction proceeded. Harden searched for some organic phosphate formed from it and located that in the form of a sugar molecule to which two phosphate groups had become attached. This was the beginning of the study of "intermediary metabolism"; the search for the numerous compounds formed as intermediates (sometimes very briefly lived ones) in the course of the chemical reactions going on in living tissue.
Some of the main lines of this search can be sketched out. The German biochemist Otto Fritz Meyerhof (1884-1951), in 1918 and the years thereafter, showed that in muscle contraction, glycogen (a form of starch) disappeared, while lactic acid appeared in corresponding amounts. In the process, oxygen was not consumed, so that energy was obtained without oxygen. Then, when the muscle rested after work, some of the lactic acid was oxidized (molecular oxygen being then consumed to pay off an "oxygen debt"). The energy so developed made it possible for the major portion of the lactic acid to be reconverted to glycogen. The English physiologist, Archibald Vivian Hill (1886- ), came to the same conclusions at about the same time, by making delicate measurements of the heat developed by contracting muscle.
The details of this conversion of glycogen to lactic acid were worked out during the 1930s by the Czech-American biochemists Carl Ferdinand Cori (1896- ) and his wife, Gerty Theresa Cori (1896-1957). They isolated a hitherto unknown compound from muscle tissue, glucose 1-phosphate (still called "Cori ester") and showed that it was the first product of glycogen breakdown. Painstakingly, they followed glucose 1-phosphate through a series of other changes and fitted each intermediate into the breakdown chain. One of the intermediates proved to be the sugar phosphate first detected by Harden a generation earlier.
The fact that Harden and the Coris came across phosphate containing organic compounds in their search for intermediates was significant. Throughout the first third of the twentieth century, the phosphate group was found to play an important part in one biochemical mechanism after another. The German-American biochemist, Fritz Albert Lipmann (1899- ), explained this by showing that phosphate groups could occur within molecules in one of two types of arrangement: low energy and high energy. When molecules of starch or fat were broken down, the energy liberated was used to convert low-energy phosphates to high-energy phosphates. In this way, the energy was stored in convenient chemical form. The breakdown of one high-energy phosphate liberated just enough energy to bring about the various energy-consuming chemical changes in the body.
Meanwhile, those steps in the breakdown of glycogen that lay beyond lactic acid and that did require oxygen could be studied by means of a new technique developed by a German biochemist Otto Heinrich Warburg (1883- ). In 1923, he devised a method for preparing thin slices of tissue (still alive and absorbing oxygen) and measuring their oxygen uptake. He used a small flask attached to a thin U-shaped tube. In the bottom of the tube was a colored solution. Carbon dioxide produced by the tissue was absorbed by a small well of alkaline solution within the flask. As oxygen was absorbed without being replaced in the air by carbon dioxide, a partial vacuum was produced in the flask and the liquid in the U-tube was sucked upward toward the flask. The rate of level change of the fluid, measured under carefully controlled conditions, yielded the rate of oxygen uptake.
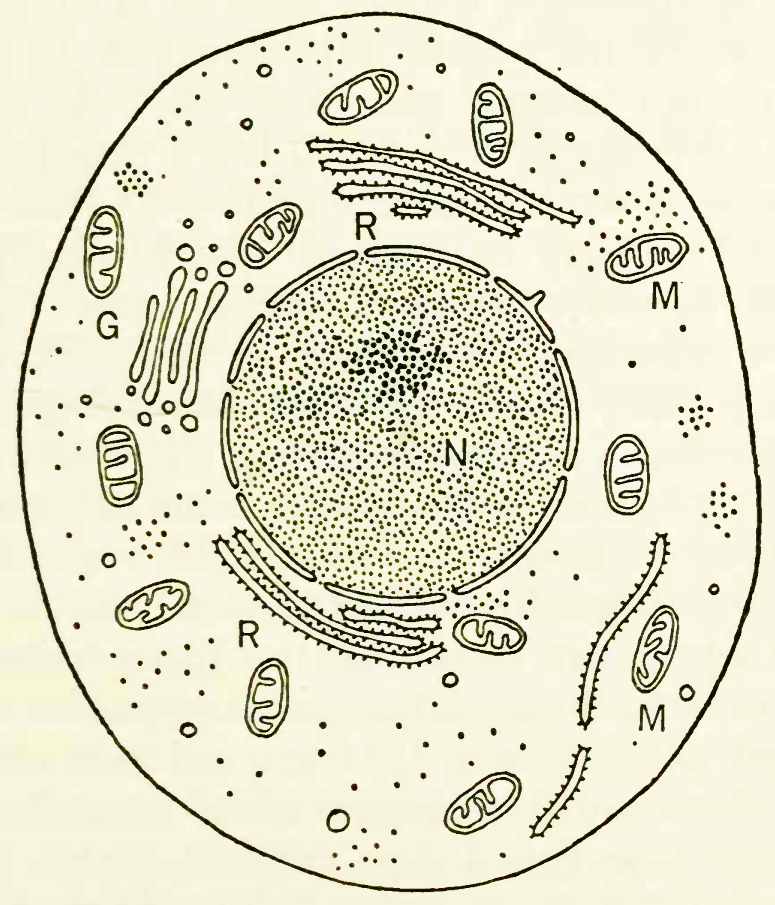
The generalized structure of a cell, seen through an electron microscope. N is the nucleus, the darker area within that the nucleolus; M denotes the mitochondria, G the Golgi bodies, and R the reticulum of particle-covered membranes. The dots here and elsewhere in the cell represent centers of protein synthesis and are known as ribosomes.
The influence of different compounds on this rate of uptake could then be studied. If a particular compound restored the rate after it had fallen off, it might be taken to be an intermediate in the series of reactions involved in oxygen uptake. The Hungarian biochemist, Albert Szent-Gyorgyi (1893- )andthe German-British biochemist, Hans Adolf Krebs (1900- ), were active in this respect. Krebs had, indeed, by 1940, worked out all the main steps in the conversion of lactic acid to carbon dioxide and water, and this sequence of reactions is often called the "Krebs cycle." Earlier, during the 1930s, Krebs had also worked out the main steps in the formation of the waste product, urea, from the amino acid building blocks of proteins. This removed the nitrogen and the remainder of the amino acid molecules could, as Rubner had shown almost a half-century earlier, be broken down to yield energy.
Hand in hand with this increase of knowledge concerning the internal chemistry of the cell came an increase of knowledge concerning the fine structure of the cell. New techniques for the purpose were developed. In the early 1930s, the first "electron microscope" was built. This magnified by focusing electron beams rather than light waves and the result was far greater magnification than was possible with ordinary microscopes. The Russian-American physicist, Vladimir Kosma Zworykin (1889- ), modified and refined the instrument to the point where it became a practical and useful tool in cytology. Particles no larger than very large molecules could be made out and the protoplasm of the cell was found to be an almost bewildering complex of small but highly organized structures called "organelles" or "particulates."
Techniques were devised, in the 1940s, whereby cells would be minced up and the various organelles separated according to size. Among the larger and more easily studied of these are the "mitochondria" (singular, "mitochondrion"). A typical liver cell will contain about a thousand mitochondria, each a rodlike object, about two to five thousandths of a millimeter long. These were investigated in particular detail by the American biochemist, David Ezra Green (1910- ), and his associates and were found by them to be the site of the reactions of the Krebs cycle. Indeed, all the reactions involving the use of molecular oxygen took place there, with the enzymes catalyzing the various reactions arranged in appropriate organization within each mitochondrion. The little organelle thus proved to be "the powerhouse of the cell."
Date added: 2023-02-03; views: 658;
