Iron Functions in the Body
Iron is a key component of flavonoids, substances that transfer electrons in the final stage of body energy production; it also intervenes in cellular replication, participates in the immune system, is essential during early life for the growth and development of the spinal cord and the brain, and acts as a cofactor participating in the activity of numerous enzymes related to the synthesis of neurotransmitters and desoxyribonucleic acid (DNA).
Thus, iron is vital for cellular functioning, although it is also known to be potentially toxic for the cells. Because of this, the body has a complex system that maintains the body stores of iron under very tight control, facilitating the accretion of iron while preventing the excessive accumulation of this mineral.
Human beings get iron mainly from food, and its intestinal absorption is regulated by the actual body stores of iron, the iron content in food, and the balance between promoter and inhibitor factors of iron absorption.
There are two types of iron available in the diet: Hematinic and nonhematinic iron. The former is present only in animal tissues, i.e., red meat, pork, fish, poultry, and viscera. The latter is found in milk, eggs, cereals, legumes, and vegetables. Additionally, the use of iron- made pots and pans for cooking may increase the content of iron in food, particularly of those foods with an acid pH (Kapur et al, 2002; Galloway, 2003a, 2003b; WHO/ UNICEF, 2005).
The quantity of both types of iron absorbed by the body depends on the content of iron in the diet, the balance between dietary enhancers and inhibitors of iron absorption, and the size of the body stores (Galloway, 2003a, 2003b; WHO/UNICEF, 2005) (Table 4).

Table 4. Substances that inhibit and enhance absorption of iron
Iron is absorbed in an inverse proportion to the size of the body stores, hematinic from 15% in normal individuals to 35% in those with iron deficiency. Nonhematinic iron absorption varies from 8% with the presence of enhancer factors to roughly 3% without their presence.
In the long run, a low content of iron in the diet or its inadequate absorption causes a reduction of the body’s production of red cells and hemoglobin. During periods of fast growth such as fetal growth, the first 2 years of life, and adolescence, the red cell mass grows; therefore the body’s iron requirements increase accordingly (Galloway, 2003a, 2003b).
When iron stores are not sufficient to carry out cellular functions properly, individuals are in a negative balance, which is divided into four stages. In stage I, low availability of iron results in depleted stores of this mineral. In stage II, the concentration of serum ferritin, the main storage protein for iron, decreases, but no alterations in the hemoglobin concentrations are seen.
Stage III is characterized by an insufficiency of iron for erythropoiesis, which decreases saturation of iron in transferrin, the main plasma transporter of iron, with a consequent increase in the number of serum transferrin receptors and erythrocytic free protoporphyrin concentrations. In stage IV, the depletion of iron stores and the reduction of its circulating concentrations persist, which is associated with a reduction in the concentration of circulating hemoglobin and the size of erythrocytes and their hemoglobin content. Such alterations are reflected in smaller red cells (microcytosis) with a paler color (hypochromia). This last stage is known as iron deficiency anemia (Beard, 2001; Galloway, 2003a, 2003b; WHO/UNICEF, 2005).
It should be noted that the level of iron depletion observed in stages I and II (with no alteration of hemoglobin levels) is reversible provided that iron is supplemented. Individuals at these stages do not develop functional changes.
Individuals with iron deficiency at stage IV usually do not experience any symptoms; however, if anemia persists, they may show some symptoms, such as fatigue, lassitude, extreme fatigue after exercising, pale mucosa, palpitations (awareness of heart throbbing), tinnitus (hissing or ringing in the ears or head), headache, irritability, dizziness, weakness, and alterations in the immune and the thermoregulatory systems. Older persons may also develop cardiac pain (angina) and cardiac failure.
When anemia is caused by cobalamin or folate deficiency, patients may show some other symptoms besides those mentioned above, particularly a sore mouth and tongue, which may be due to a possible lack of renovation of scaly cells.
The impact of anemia goes beyond its clinical manifestations; for example, fatigue may have negative consequences on an individual’s productivity and have economic consequences. A review of the literature found that in subjects with anemia, a 10% increase in hemoglobin concentrations was associated with a 10-20% increase in work performance (Galloway, 2003a, 2003b).
It has been suggested that IDA in women of childbearing age is caused mainly by an inadequate diet, poor social and economic conditions (Figure 1), and increased iron requirements due to the rapid expansion of blood volume and the development of the fetus and the placenta. Pregnant women with anemia are 3.5-fold more likely to die of complications during and after childbirth than nonanemic women. Additionally, fatigue due to anemia makes delivery more difficult, which may be life-threatening (Galloway, 2003a, 2003b); 40% of perinatal deaths are related to anemia.
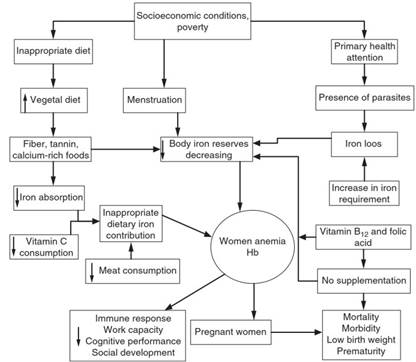
Figure 1. Determinants of anemia in women of childbearing age. Source: Shamah T and Villalpando S, 2002, (unpublished)
Although the level of hemoglobin at which anemia starts affecting the health of the mother and her newborn has not been clearly established (Beard, 2000; Rush, 2000), it is clear that acute anemia increases the risk of maternal mortality (Rush, 2000; Grantham-McGregor and Ani, 2001). There is a negative relationship between maternal hemoglobin levels and intrauterine fetal growth delay, premature birth, and low birth weight, resulting in lower survival (Galloway, 2003a, 2003b, 2004). Inadequate iron stores in the mother also result in low iron stores in the newborn. Thus, the infant will develop earlier and have more severe iron deficiency during the first months of life.
The risk of premature delivery is closely related to maternal hemoglobin levels and stage of pregnancy. A study of a cohort of 173 031 pregnant women in the United States, 96% of whom belong to the Special Supplemental Nutrition Program for Women, Infants, and Children, found that the risk of premature birth was 10-30% higher in women with low hemoglobin levels during the first and the second trimesters of pregnancy, compared with women with normal levels of hemoglobin at comparable pregnancy stages.
Women with mild anemia have 30-40% greater risk, while those with moderate to severe anemia have up to 70% higher risk for premature delivery. Low levels of hemoglobin during the third trimester of pregnancy are not associated with preterm births (Scanlon et al., 2000). Similar findings have been reported in other studies, confirming that the association between anemia and the risk of premature birth or low birth weight is greater when anemia occurs early in the gestation period than when it occurs in the last trimester of pregnancy (Scholl, 2005).
On the other hand, it has been observed that high levels of hemoglobin during pregnancy may also lead to unwanted outcomes (Scanlon etal, 2000). The same study of the cohort of pregnant women in the United States (Scanlon etal, 2000) found that women with high levels of hemoglobin during the first and second trimester of pregnancy had a 30-40% higher risk of delivering a child small for the gestational age than women with normal hemoglobin concentrations.
During a normal pregnancy without anemia, the fetus accumulates iron during the last weeks of gestation, in such an amount that the infant will fulfill his iron needs for the first 4-6 months of extrauterine life, provided exclusive breastfeeding, which supplies the infant with highly bioavailable iron. However, fetuses of anemic women will develop limited iron stores; therefore, they will be more likely to develop IDA early in the first months of life, a period characterized by rapid growth requiring a fast expansion of the blood volume.
During the first 2 years of life, the iron requirements of the human body increase progressively in order to carry out multiple functions related to the development of the brain, the immune and endocrine systems, etc. Several studies have found an association between anemia and growth delay, low cognitive, motor, and behavioral development, as well as reduced resistance to infections (Beard and Tobin, 2000). Unfortunately, the effects of anemia during the first 2 years of life are nonreversible.
In their first year of life, 10-20% of infants in industrialized countries and approximately 30-80% in nonindustrialized countries have anemia and will suffer from impaired motor development characterized by deficiencies in language, motor, and coordination skills, with a five- to ten-point deficit in their intellectual quotient (IQ) (Grantham-McGregor and Ani, 2001; Galloway, 2003a, 2003b).
At any stage of life, IDA damages the capacity of the body to regulate internal temperature. It may encompass alterations in hormone production and in energy metabolism, affecting the synthesis of neurotransmitters and thyroid hormones related to muscular and neurological functions involved in the regulation of body temperature.
Date added: 2024-03-11; views: 674;
