Arbovirus Cycles. Diversity of Arboviruses
A typical arbovirus cycle is presented in Figure 1. For an arbovirus to be successfully amplified in nature, the following conditions must be satisfied: (1) a susceptible vertebrate host (or hosts) must be present, possibly a mammal, bird, reptile, or amphibian; (2) during infection of the vertebrate, a viremic phase must ensue (i.e., a time when the virus is present in the blood stream in sufficient titer, or quantity, and of sufficient duration to be capable of infecting an arthropod when it is taking a blood meal); and (3) the virus in question must be capable of replicating in the species of arthropod taking the blood meal.
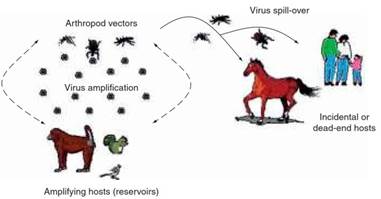
Figure 1. Typical arbovirus transmission cycle. Source: Bres P (1988) Impact of arboviruses on human and animal health. In: Monath TP (ed.) The Arboviruses: Epidemiology and Ecology, vol.1, pp. 1–18. Boca Raton, FL: CRC Press
The general pattern of arthropod infection involves initial growth of virus in the cells of the arthropod gut after ingestion of an infected blood meal. This is followed by distribution of the virus to various parts of the body by way of hemolymph (arthropod blood). Generally this occurs without any significant deleterious effect on the arthropod host.
If the arthropod is capable of transmitting the virus, virus replication must occur in the salivary glands. It is only when virus infection of the arthropod has progressed to the stage where the salivary glands are infected that the arthropod is capable of transmitting virus. The time it takes between initial infection of the arthropod and progression to the salivary gland is called the extrinsic incubation period, and is dependent on environmental factors such as temperature as well as the virus and vector species.
Some infected arthropods are capable of maintaining arboviruses without involvement of vertebrate hosts by transovarial transmission (e.g., transmission of La Crosse virus from infected female to eggs of the mosquito, Aedes triseriatus). Vertebrate-to-vertebrate transmission of arboviruses is rare but has been documented (e.g., transmission of West Nile virus can occur between infected birds).
Vertebrates that contribute to successful arbovirus cycles are called amplifying hosts. Those that are not infected or do not develop sufficient viremia to infect more arthropods are called incidental or dead-end hosts. Humans are usually incidental hosts for most arboviruses. However, for arboviruses such as dengue and Chikungunya, people serve as the principal amplifying host. Disease may or may not occur in amplifying and incidental hosts.
Susceptibility to virus infection varies greatly among arthropods as it does in vertebrates with certain species of arthropods such as A. aegypti and A. albopictus being particularly efficient vectors. In areas where arboviruses are circulating, it is often possible to isolate arboviruses from many different species of arthopods including some that do not have an important role in the amplification cycle of the arbovirus in question.
This spillover to other species can include bridging vectors that may play an important role in transmitting viruses to humans. For example, eastern equine encephalitis virus is maintained in a bird-mosquito transmission cycle in Culiseta melanura, a mosquito species that feeds almost exclusively on birds.
Transmission to humans occurs when certain Aedes, Coquillettidia, and Culex species feed upon birds and then humans, thus acting as a ‘bridging’ vector between the enzootic (affecting a limited number of animals in a specific geographic area) and epizootic/epidemic (increased number of cases in animals/humans) cycles.
Diversity of Arboviruses. Arboviruses may have markedly different biological properties and belong to at least eight different families of viruses (Figure 2). However, the majority of arboviruses are single-stranded, spherical, enveloped RNA viruses belonging to one of three families called Flaviviridae, Togaviridae, and Bunyaviridae.
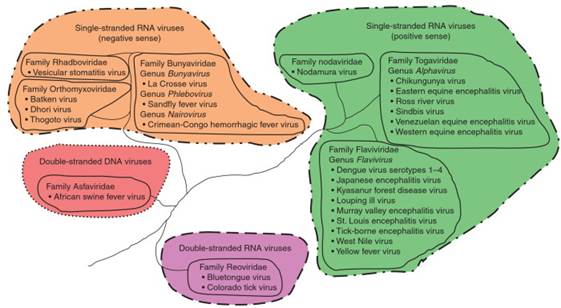
Figure 2. Biodiversity of arboviruses
The family Togaviridae contains the alphavirus genus consisting of 27 viruses distributed worldwide. The occurrence of alphaviruses is mainly limited to the Southern Hemisphere. All alphaviruses of medical importance are transmitted to humans by mosquitoes. More than one mosquito species is usually involved in an alphavirus cycle, and bird migration often plays a role in the importation of alphaviruses into countries in the Northern Hemisphere.
Important vertebrate-amplifying hosts include: birds (eastern equine encephalitis, Sindbis, Sem-liki Forest, western equine encephalitis), rodents (Barmah Forest, Ross River, Venezuelan equine encephalitis), and primates (Chikungunya, Mayaro, O’nyong-nyong). Clinical manifestations generally include encephalitis and febrile disease often with joint involvement. Diseases caused by alphaviruses tend to be more severe in children.
The family Flaviviridae includes the Flavivirus genus that has at least 74 viruses with a worldwide distribution. There are approximately 40 mosquito-borne and 16 tick- borne viruses, and 18 viruses for which an arthropod vector is not known. Birds are the most important vertebrate hosts for those flaviviruses that are transmitted by mosquitoes that belong to the Japanese encephalitis complex. However, simians and humans are the important hosts for mosquito-transmitted dengue and yellow fever viruses.
Rodents are the most important hosts for tick- transmitted flaviviruses. Some of the most relevant human-disease-causing arboviruses belong to this genus and produce a wide range of symptoms including fever, arthralgia, rash, hemorrhagic fever, and encephalitis. Examples of flaviviruses of medical importance include dengue, yellow fever, Japanese encephalitis, West Nile, St. Louis encephalitis, Murray Valley encephalitis, and tick-borne encephalitis (TBE) viruses. Diseases caused by flaviviruses tend to be more severe in the elderly.
The family Bunyaviridae constitutes the largest family of viruses with over 300 viral species in five genera. Three genera, Bunyavirus, Nairovirus, and Phlebovirus, contain viruses capable of causing disease in humans and are transmitted in nature by different types of arthropods including mosquitoes, ticks, and sandflies.
Arthropod-transmitted bunyaviruses are closely adapted to their vectors and can survive without involvement of a vertebrate host by transovarial (female arthropod to progeny) transmission under unfavorable climatic conditions. Members of the Bunyaviridae family can be found worldwide with approximately 20 viruses capable of causing clinical disease in humans.
Manifestations include: fever (sometimes hemorrhagic), renal failure, encephalitis, meningitis, and blindness. Examples of bunyaviruses of medical importance include Crimean-Congo hemorrhagic fever, Rift Valley fever, and La Crosse, Naples, and Sicilian sandfly fever viruses.
Other families of viruses contain smaller numbers of arboviruses and are of less significance from a human health perspective but do reflect the great diversity of arboviruses. The family Rhabdoviridae has certain viruses within the genus Vesiculovirus that are proven or probable arboviruses with sandflies acting as the significant arthropod vectors for this group of viruses.
The family Reoviridae, a virus family with a double-stranded RNA genome as opposed to the single-stranded RNA arboviruses noted previously, contains a few tick-transmitted human pathogens including Colorado tick fever and Kemerovo viruses. Finally, there is one double-stranded DNA arbovirus, African swine fever virus, a tick-transmitted virus belonging to the family Asfaviridae that causes disease in swine.
Date added: 2024-03-11; views: 787;
